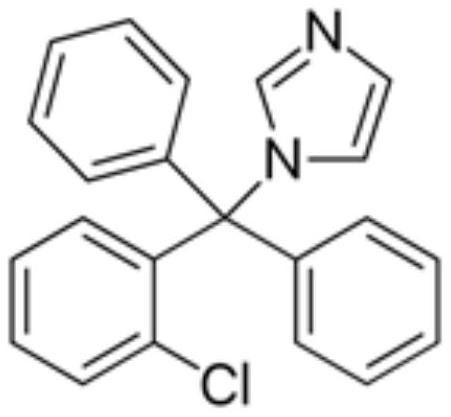
Single-dose packaged clotrimazole liquid composition
A single-dose, composition-based technology used in the medical and pharmaceutical packaging industry to address issues of lack of stability, loss of efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1 - PEG solution (1.2% water content) of a single dose package
[0138] Formula 1 :
[0139] Element % (W / v) Gramps 1% Polyethylene glycol 400 (PEG-400) Mandidity is 100%
[0140] Manufacturing process :
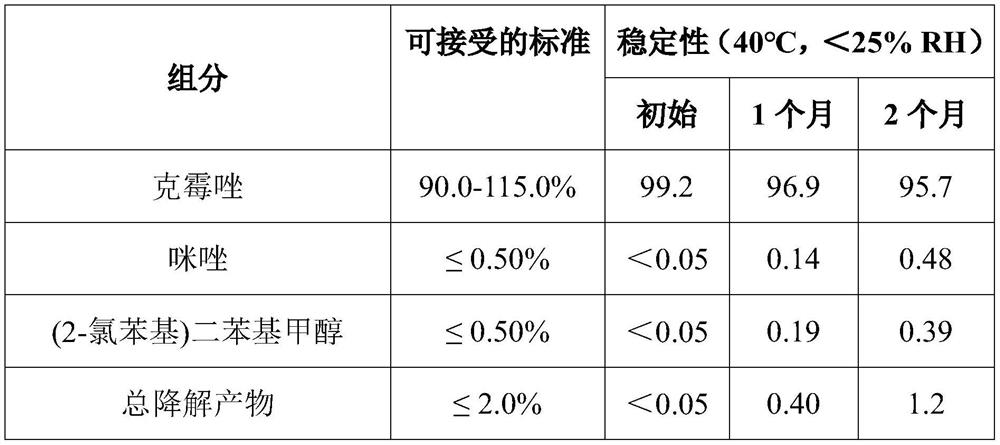
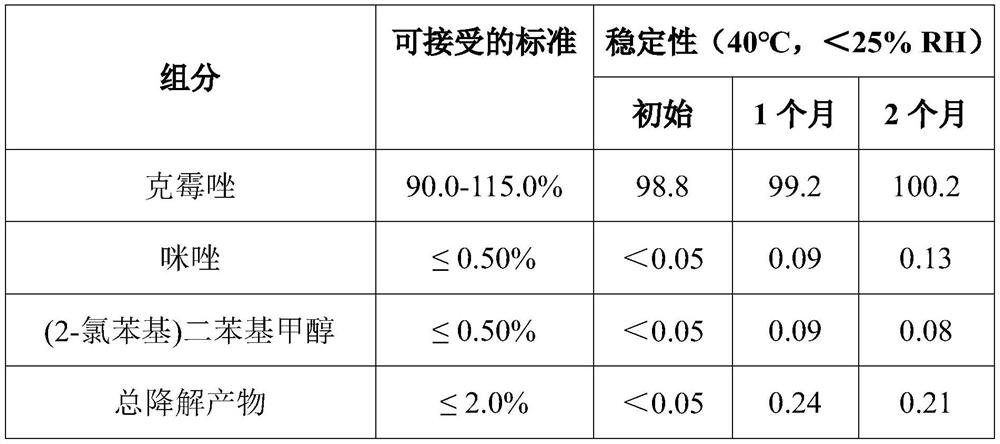
[0141] The single dose package of this embodiment is obtained in the BFS Equipment Rommelag BP321M. First, a solution of the active ingredient in PEG 400 was prepared at 40 ± 15 ° C. In order to sterilize the solution, two sterilized filters (polyvinylidene fluoride (PVDF) membrane filters, aperture of 0.22 μm) were used. The integrity of these filters was checked prior to use and dried at 70 ° C for at least 5 hours. On the other hand, the storage tank was sterilized with vapor and the residual water was removed after sterilization. The grade p-PEG solution was filtered through the first drying filter. The first volume filtering solution was discarded, and the filtered solution was introduced into the first storage tank after minimizi...
Embodiment 2
[0142] Example 2 - Single dose packaged gramps PEG solution (0.8% water content, oxygen content
[0143] Formula 1 :
[0144] Element % (W / v) Gramps 1% Polyethylene glycol 400 (PEG-400) Mandidity is 100%
[0145] Manufacturing process :
[0146] As described in Example 1, the single dose package of Example 2 was prepared in Example 2, in addition to the fact that a nitrogen stream is used during seal secondary packaging (aluminum foil composite bag). Compared to total weight of the composition, the water content of the product (by Karl Fischer USP 41 (Method I, instrument Volumetric Karl Fischer TitRatorTler Toledo V20s) is 0.8%, and the total volume of gas content in the secondary package, Oxygen content in secondary packaging (through top aerodynamic analyzer Measurement) below 10% by volume.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com