Compound preparation for treating hyperuricemia and gout
A hyperuricemia and preparation technology, applied in the field of medicine, can solve problems affecting organ metabolism and fatal adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
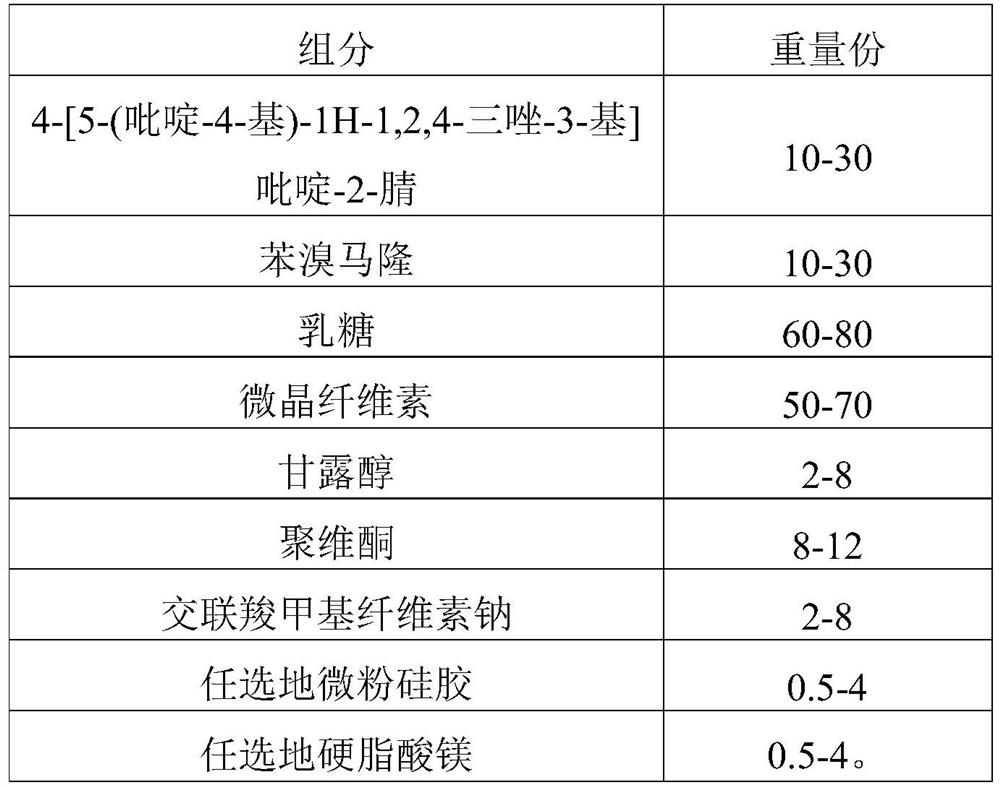
[0190] The present embodiment 1 provides a kind of compound tablet and preparation method thereof, and described compound tablet prescription is as shown in table 1 below:
[0191] Table 1 Compound Tablet Prescription (1000 Tablets)
[0192]
[0193] The preparation method of the compound tablet of the present embodiment 1
[0194] 1. Adhesive preparation: the povidone of prescription quantity is mixed with water and is prepared into 5% (mass concentration) povidone aqueous solution, as adhesive.
[0195] 2. Weighing and sieving: take the active ingredients of each prescription amount and auxiliary materials except magnesium stearate and povidone respectively, and pass through a 20-mesh sieve to obtain raw and auxiliary materials.
[0196] 3. Pre-mixing: Add the above raw and auxiliary materials into the hopper mixer and pre-mix for 15 minutes.
[0197] 4. Fluidized bed granulation: put the pre-mixed material into the fluidized bed, and use 5% povidone aqueous solution as...
Embodiment 2
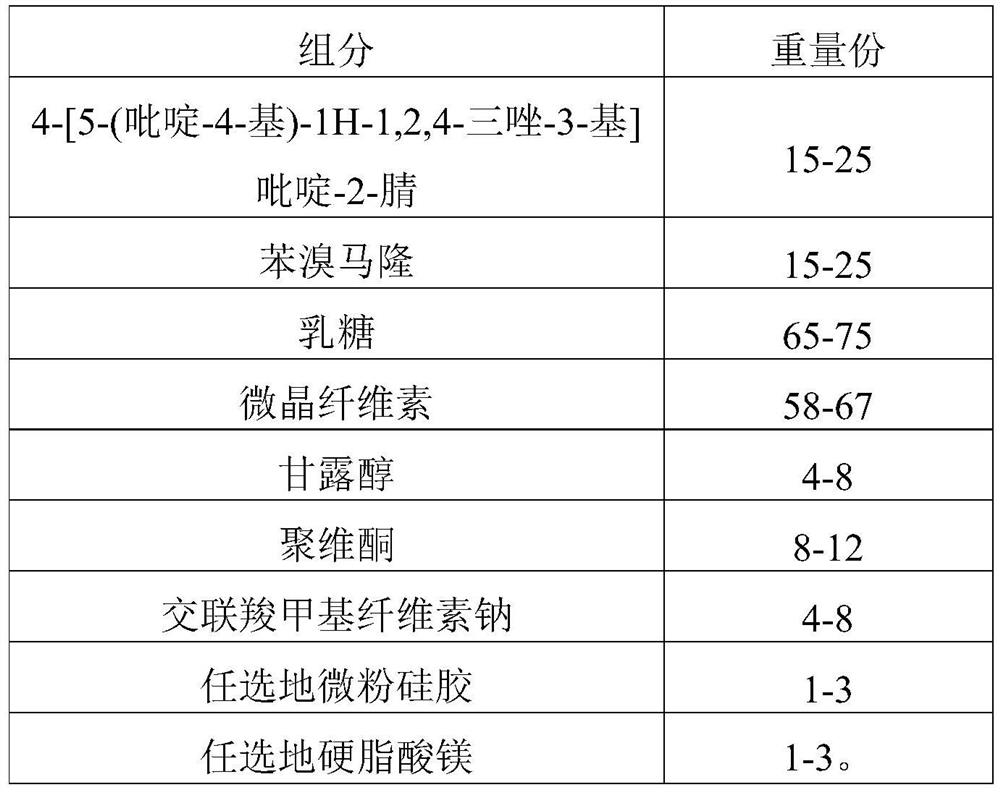
[0202] Present embodiment 2 provides a kind of compound tablet and preparation method thereof, and described compound tablet prescription is as shown in table 2 below:
[0203] Table 2 Prescription of Compound Tablets (1000 Tablets)
[0204]
[0205] The compound tablet preparation method of present embodiment 2
[0206] 1. Adhesive preparation: the povidone of prescription quantity is mixed with water and is prepared into 5% (mass concentration) povidone aqueous solution, as adhesive.
[0207] 2. Weighing and sieving: take the active ingredients of each prescription amount and auxiliary materials except magnesium stearate and povidone respectively, and pass through a 20-mesh sieve to obtain raw and auxiliary materials.
[0208] 3. Pre-mixing: Add the above raw and auxiliary materials into the hopper mixer and pre-mix for 15 minutes.
[0209] 4. Fluidized bed granulation: put the pre-mixed material into the fluidized bed, and use 5% povidone aqueous solution as the binder...
Embodiment 3
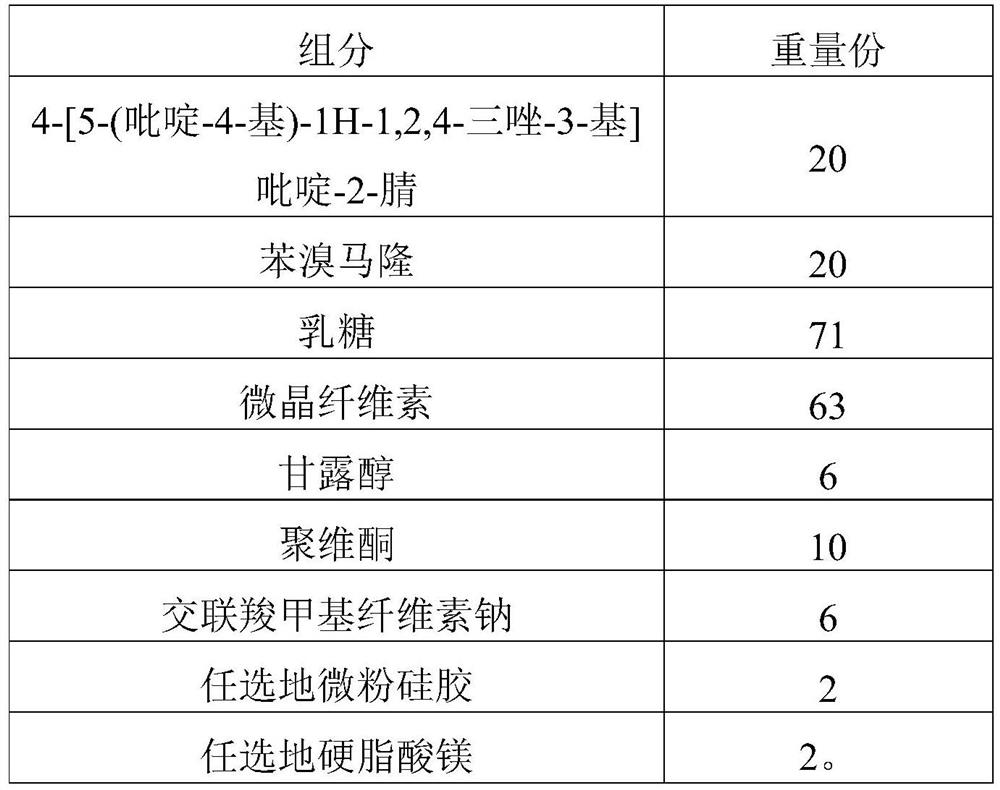
[0214] Present embodiment 3 provides a kind of compound tablet and preparation method thereof, and described compound tablet prescription is as shown in table 3 below:
[0215] Table 3 Prescription of Compound Tablets (1000 Tablets)
[0216]
[0217] The compound tablet preparation method of present embodiment 3
[0218] 1. Adhesive preparation: the povidone of prescription quantity is mixed with water and is prepared into 5% (mass concentration) povidone aqueous solution, as adhesive.
[0219] 2. Weighing and sieving: take the active ingredients of each prescription amount and auxiliary materials except magnesium stearate and povidone respectively, and pass through a 20-mesh sieve to obtain raw and auxiliary materials.
[0220] 3. Pre-mixing: Add the above raw and auxiliary materials into the hopper mixer and pre-mix for 15 minutes.
[0221] 4. Fluidized bed granulation: put the pre-mixed material into the fluidized bed, and use 5% povidone aqueous solution as the binder...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com