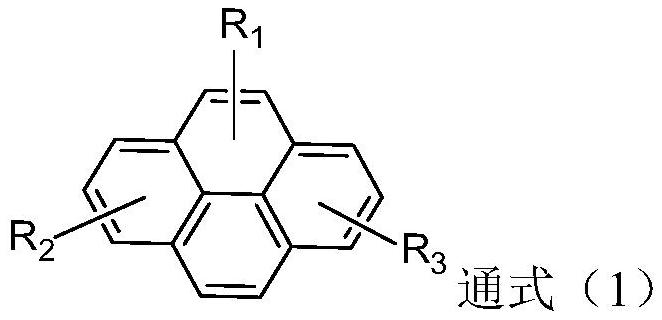
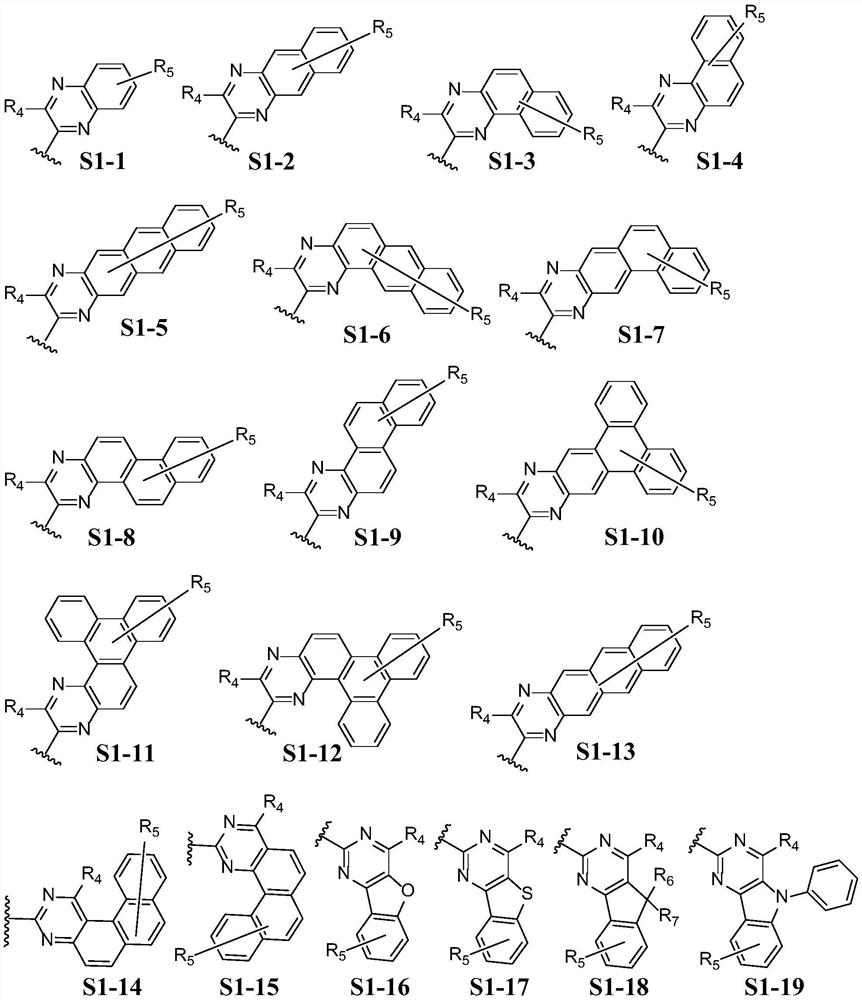
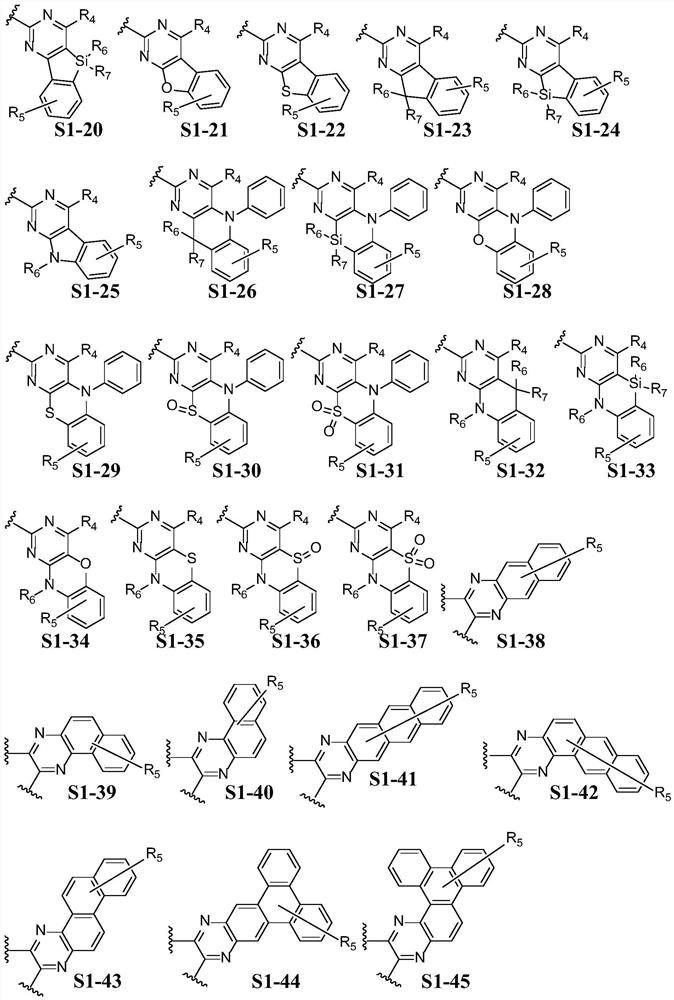
Pyrene derivative, luminescent device material and luminescent device
A technology for derivatives and optoelectronic devices, applied to pyrene derivatives, can solve problems such as lack of materials, and achieve the effects of easy availability of raw materials, reduction of vibration energy loss, and high-efficiency luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Synthesis of compound 1
[0092]
[0093] Under an argon atmosphere, 39.4 g (100 mmol) of 1,8-dibromo-4-chloropyrene, 42.3 g (200 mmol) of dibenzo[b, d]furan-3-ylboronic acid, tetrakis(tri Phenylphosphine) palladium 1.16g (1.0mmol), 1.5M aqueous sodium carbonate solution 200ml (300mmol) and ethylene glycol dimethyl ether 800ml (DME), heated and stirred at 80°C overnight. Cool to room temperature, add 500ml of water, solid precipitates, filter, and the obtained solid is washed with ethanol to obtain 37.0g of 3,3'-(4-chloropyrene-1,8-diyl)dibenzo[b,d ] furan, yield 65%, HPLC purity 98.5%.
[0094] 1 HNMR(DMSO): δ8.52(d, 1H), 8.30(d, 1H), 8.15(d, 2H), 8.03~7.98(m, 4H), 7.82~7.76(m, 4H), 7.72~7.70( m,3H),7.54(m,2H),7.39~7.31(m,4H)
[0095]
[0096] Under an argon atmosphere, 56.9 grams (100 mmol) of 3,3'-(4-chloropyrene-1,8-diyl)dibenzo[b,d]furan, (3-phenylquinoxa Lin-2-yl)boronic acid 30.0g (120mmol), [1,3-bis(2,6-di-isopropylphenyl)-4,5-dihydroimidazol-2-yliden...
Embodiment 2
[0099] Synthesis of Compound 21
[0100]
[0101] Under argon atmosphere, 31.6g (100mmol) of 1-bromo-4-chloropyrene, 36.0g (100mmol) of 3-boronic acid-9,9'-spirobifluorene, tetrakis(triphenylphosphine) palladium 1.16g (1.0mmol), 200ml (300mmol) of 1.5M aqueous sodium carbonate solution and 800ml of ethylene glycol dimethyl ether (DME), heated and stirred at 80°C overnight. Cooled to room temperature, added 500ml of water, solid precipitated, filtered, and the obtained solid was washed with ethanol to obtain 38.6g of 3-(4-chloropyren-1-yl)-9,9'-spiro[fluorene]. Yield 70%, HPLC purity 98.9%.
[0102] 1 HNMR(DMSO): δ8.30(d, 1H), 8.18~8.15(m, 2H), 8.07~8.04(m, 2H), 7.92~7.89(m, 5H), 7.74~7.68(m, 4H), 7.55(d, 1H), 7.45(m, 2H), 7.38(m, 1H), 7.28~7.27(m, 5H)
[0103]
[0104] Under an argon atmosphere, 55.1 grams (100 mmol) of 3-(4-chloropyren-1-yl)-9,9'-spiro[fluorene], (3-phenylquinoxaline-2- Base) boronic acid 30.0g (120mmol), [1,3-bis(2,6-di-isopropylphenyl)-4,5-dihydr...
Embodiment 3
[0106] Synthesis of compound 27
[0107]
[0108] Under argon atmosphere, 39.4g (100mmol) of 1,8-dibromo-4-chloropyrene and 47.6g (200mmol) of (9,9-dimethyl-9H-fluoren-1-yl)boronic acid were added to the reactor 1.16g (1.0mmol) of tetrakis(triphenylphosphine)palladium, 200ml (300mmol) of 1.5M aqueous sodium carbonate solution and 1000ml of ethylene glycol dimethyl ether (DME), heated and stirred at 80°C overnight. Cool to room temperature, add 500ml of water, solid precipitates, filter, and wash the obtained solid with ethanol to obtain 44.1g of 4-chloro-1,8-bis(9,9-dimethyl-9H-fluoren-1-yl) Pyrene, yield 71%, HPLC purity 98.8%.
[0109] 1 HNMR(DMSO): δ8.52(d, 1H), 8.30(d, 1H), 8.15(d, 2H), 8.00(m, 2H), 7.90(m, 2H), 7.72~7.68(m, 5H) ,7.57~7.55(m,4H),7.38(m,2H),7.28(m,2H),1.69(s,12H).
[0110]
[0111] Under an argon atmosphere, 62.1 grams (100 mmol) of 4-chloro-1,8-bis(9,9-dimethyl-9H-fluoren-1-yl)pyrene, (3-phenylquinoxaline -2-yl)boronic acid 30.0g (120mmol), [1,3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com