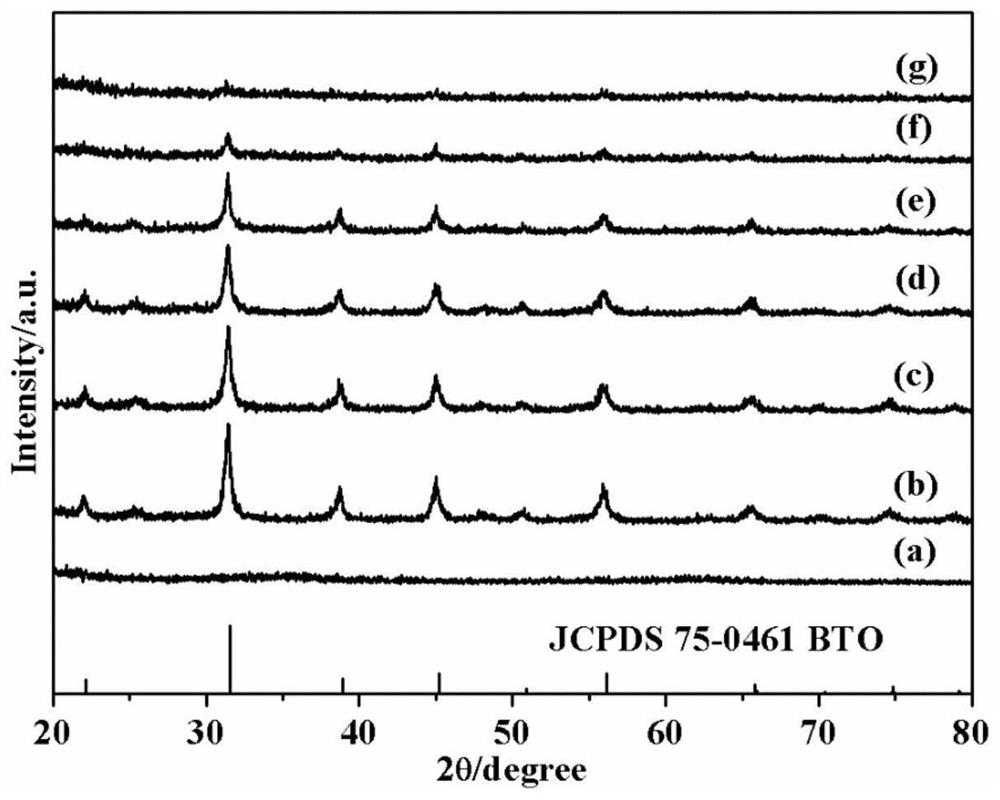
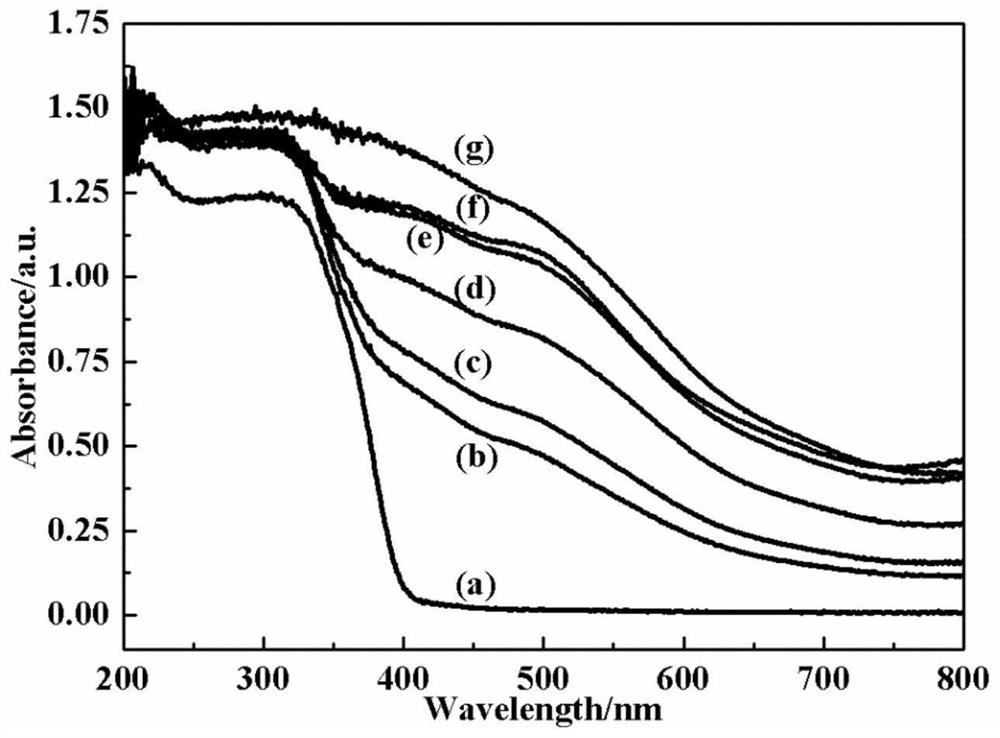
Preparation method of barium titanate/iron oxyhydroxide photocatalyst
A technology of iron oxide light and barium titanate, which is applied in the field of photocatalysis, can solve the problems of waste of resources, narrow pH application range, etc., and achieve the effect of promoting separation, short time, and expanding the spectrum absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A kind of preparation method of barium titanate / iron oxyhydroxide photocatalyst, comprises the following steps:
[0033] Step 1: Add 18.3ml of N,N-dimethylacetamide (DMAC) to 58.4mL of isopropanol (IPA) and stir for a period of time, then add 2.5ml of tetrabutyl titanate (TBOT ), magnetically stirred evenly. Then pour the obtained suspension into a 100ml hydrothermal kettle, set the oven parameters to 200 ° C for 20 hours, and separate the suspension after the reaction is completed, wash it with absolute ethanol, and finally dry it in a 60 ° C oven to obtain H 2 Ti 2 o 5 Precursor;
[0034] Step 2: Pour 7ml of water into 28ml of absolute ethanol, and magnetically stir for 10min. Add 0.2g of H 2 Ti 2 o 5 Precursor, 0.3g of NaOH and 0.32g of Ba(OH) 2 ·8H 2 O was added to the above solution in turn, stirred by magnetic force for 30min, and then placed in a hydrothermal kettle for reaction. The temperature of the oven was set at 140°C, and the reaction time was set...
Embodiment 1
[0056] Step 1: Add 18.3ml of N,N-dimethylacetamide (DMAC) to 58.4mL of isopropanol (IPA) and stir for a period of time, then add 2.5ml of tetrabutyl titanate (TBOT ), magnetically stirred evenly. Then pour the obtained suspension into a 100ml hydrothermal kettle, set the oven parameters to 200 ° C for 20 hours, and separate the suspension after the reaction is completed, wash it with absolute ethanol, and finally dry it in a 60 ° C oven to obtain H 2 Ti 2 o 5 Precursor;
[0057] Step 2: Pour 7ml of water into 28ml of absolute ethanol, and magnetically stir for 10min. Add 0.2g of H 2 Ti 2 o 5 Precursor, 0.3g of NaOH and 0.32g of Ba(OH) 2 ·8H 2 O was added to the above solution in turn, stirred by magnetic force for 30min, and then placed in a hydrothermal kettle for reaction. The temperature of the oven was set at 140°C, and the reaction time was set at 8h. After the reaction, the precipitate was collected by centrifugation, washed with deionized water, and finally heat...
Embodiment 2
[0060] Step 1: Add 18.3ml of N,N-dimethylacetamide (DMAC) to 58.4mL of isopropanol (IPA) and stir for a period of time, then add 2.5ml of tetrabutyl titanate (TBOT ), magnetically stirred evenly. Then pour the obtained suspension into a 100ml hydrothermal kettle, set the oven parameters to 200 ° C for 20 hours, and separate the suspension after the reaction is completed, wash it with absolute ethanol, and finally dry it in a 60 ° C oven to obtain H 2 Ti 2 o 5 Precursor;
[0061] Step 2: Pour 7ml of water into 28ml of absolute ethanol, and magnetically stir for 10min. Add 0.2g of H 2 Ti 2 o 5 Precursor, 0.3g of NaOH and 0.32g of Ba(OH) 2 ·8H 2O was added to the above solution in turn, stirred by magnetic force for 30min, and then placed in a hydrothermal kettle for reaction. The temperature of the oven was set at 140°C, and the reaction time was set at 8h. After the reaction, the precipitate was collected by centrifugation, washed with deionized water, and finally heate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com