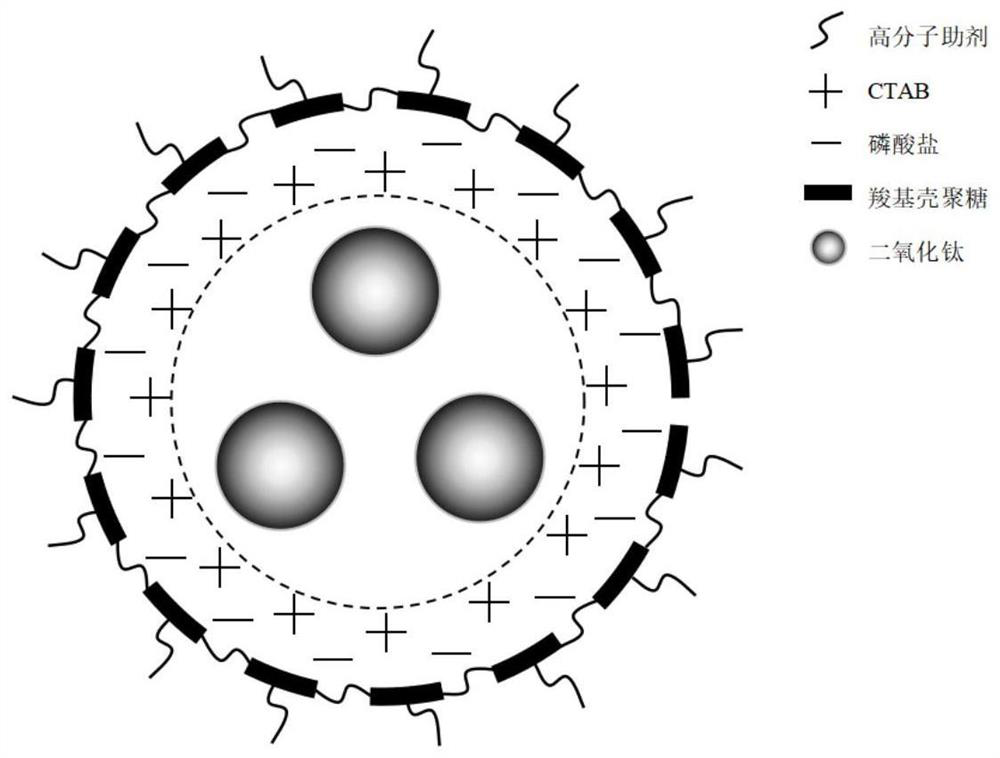
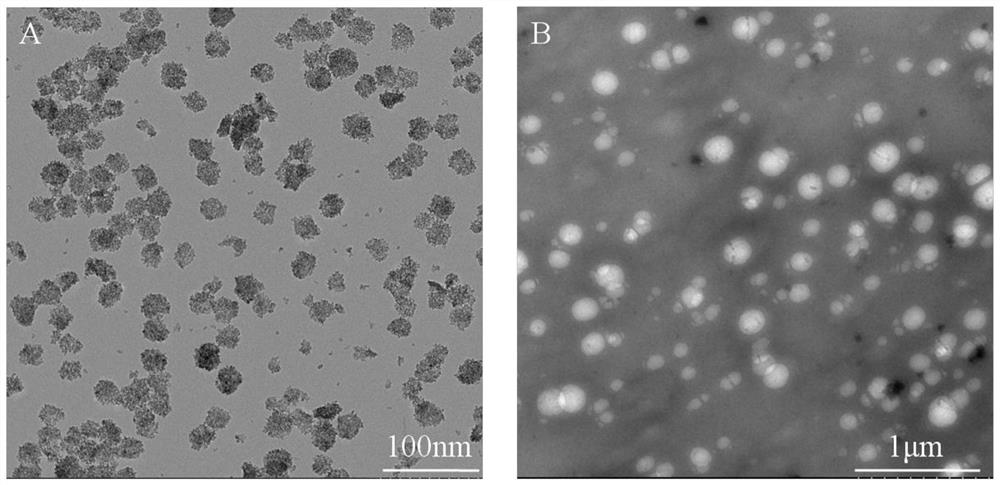
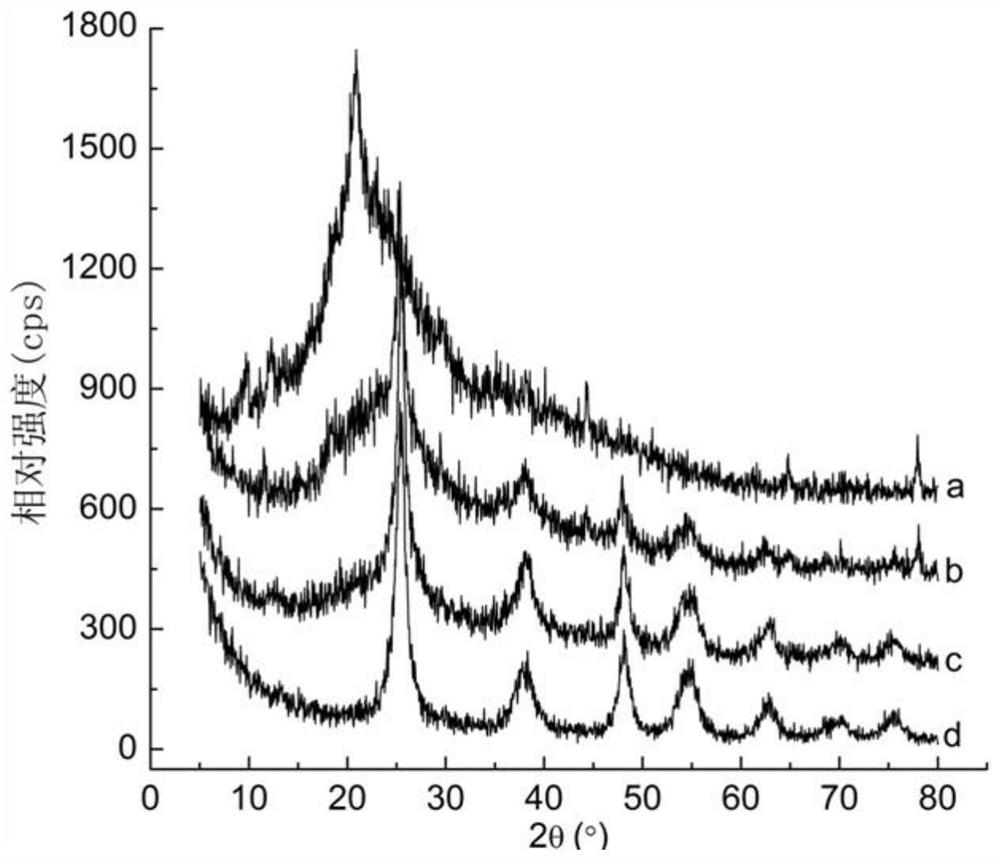
Preparation method of core-shell type titanium dioxide-coated carboxyl chitosan nanoparticles
A carboxyl chitosan, titanium dioxide technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc. to avoid hydrolysis and agglomeration, improve reaction efficiency and uniformity, and improve biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In this example, core-shell titanium dioxide@carboxychitosan nanoparticles were prepared according to the following steps:
[0038] (1) Under strong stirring, add 1mL tetrabutyl titanate dropwise to 10mL ionic liquid 1-ethyl-3-methylimidazole acetate within 5min at a constant speed, react at room temperature for 0.5h, and add 2mL dropwise at a constant speed within 5min. Ionized water, reacted at 40°C for 4 hours, added 20mL of absolute ethanol, stirred evenly, centrifuged three times at high speed, discarded the supernatant, sonicated three times with 50% ethanol, filtered, and dried in vacuum at 40°C to obtain anatase nano-titanium dioxide.
[0039] (2) Take 0.025g of titanium dioxide prepared in the above step (1) and disperse it in 50mL of 2% acetic acid (v / v) solution, add 1mmol CTAB, stir vigorously for 30min, put it into a reaction kettle, place it in a microwave reactor, and ℃ for 20 minutes to prepare a cation-modified titanium dioxide dispersion. The power of...
Embodiment 2
[0043] In this example, core-shell titanium dioxide@carboxychitosan nanoparticles were prepared according to the following steps:
[0044] (1) Under strong stirring, add 2.5mL tetrabutyl titanate dropwise to 15mL ionic liquid 1-ethyl-3-methylimidazole acetate within 10min at a constant speed, react at room temperature for 1h, and add 5mL dropwise at a constant speed within 10min to remove Ionized water, reacted at 45°C for 5 hours, added 30mL of absolute ethanol, stirred evenly, centrifuged at high speed for 3 times, discarded the supernatant, ultrasonicated 50% (v / v) ethanol three times, filtered, and dried in vacuum at 40°C to obtain anatase type nano titanium dioxide.
[0045] (2) Take 0.05g of titanium dioxide prepared in the above step (1) and disperse it in 50mL of 2% acetic acid (v / v) solution, add 3mmol CTAB, stir vigorously for 30min, put it into a reaction kettle, place it in a microwave reactor, and ℃ for 30 minutes to prepare a cation-modified titanium dioxide dis...
Embodiment 3
[0049] In this example, core-shell titanium dioxide@carboxychitosan nanoparticles were prepared according to the following steps:
[0050] (1) Under strong stirring, add 5 mL of tetrabutyl titanate to 20 mL of ionic liquid 1-ethyl-3-methylimidazole acetate at a constant speed within 15 minutes, react at room temperature for 1.5 hours, and add 8 mL at a constant speed within 20 minutes to remove Ionized water, reacted at 50°C for 6h, added 40mL of absolute ethanol, stirred evenly, centrifuged at high speed for 3 times, discarded the supernatant, ultrasonicated 50% (v / v) ethanol for three times, filtered, and dried in vacuum at 40°C to obtain anatase Nano titanium dioxide.
[0051] (2) Take 0.10 g of titanium dioxide prepared in the above step (1) and disperse it in 50 mL of 2% acetic acid (v / v) solution, add 6 mmol of CTAB, stir vigorously for 30 min, put it into a reaction kettle, place it in a microwave reactor, and ℃ for 50 minutes to prepare a cation-modified titanium diox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com