Method for producing nickel cobalt manganese sulfate by using nickel cobalt manganese hydroxide raw material
A technology of nickel hydroxide cobalt and nickel sulfate, which is applied in the direction of nickel sulfate, etc., can solve the problems of high energy consumption and cumbersome process, and achieve the effects of simplifying the production process, solving time-consuming problems, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
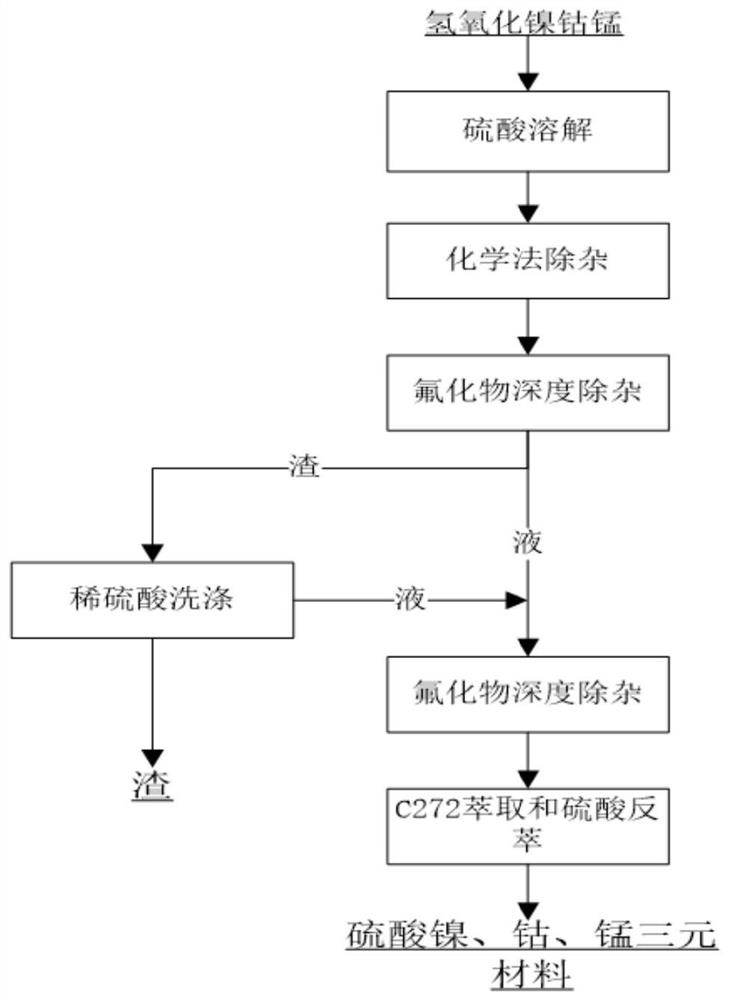
[0030] Such as figure 1 As shown, take nickel cobalt hydroxide raw material, take 1kg nickel cobalt hydroxide material as example, in this nickel cobalt hydroxide material after testing: the content of Co is 4.2%, the content of Ni is 30.3%, the content of Fe is 0.35% , the content of Al is 0.15%, the content of Ca is 0.6%, and the content of Mg is 0.6%. The above-mentioned nickel cobalt hydroxide raw material is processed as follows:
[0031] a. The above-mentioned nickel cobalt hydroxide raw material and water are made into a slurry according to the solid-to-liquid ratio of 1:2, and then the pH of the solution is adjusted to 1.5 by adding sulfuric acid, and the reaction temperature is controlled at 70°C, and the reaction time is 1h, and the nickel cobalt hydroxide raw material is Dissolved into a solution containing nickel and cobalt sulfate.
[0032] b. Remove iron, aluminum and calcium and magnesium from the solution containing nickel and cobalt sulfate obtained in step ...
Embodiment 2
[0036] Such as figure 1 As shown, get the nickel cobalt hydroxide raw material obtained by recovering the nickel cobalt raw material intermediate, take 1kg nickel cobalt hydroxide material as an example, in the nickel cobalt hydroxide material after testing: the content of Co is 5.8%, and the content of Ni is 38% , the content of Fe is 0.4%, the content of Al is 0.12%, the content of Ca is 0.5%, and the content of Mg is 0.8%. The above-mentioned nickel cobalt hydroxide raw material is processed as follows:
[0037]a. The above-mentioned nickel cobalt hydroxide raw material and water are made into a slurry according to a solid-to-liquid ratio of 1:8, and then the pH of the solution is adjusted to 0.5 by adding sulfuric acid, the reaction temperature is controlled at 65°C, and the reaction time is 0.5h, and the nickel cobalt hydroxide The raw materials are dissolved into a solution containing nickel and cobalt sulfate.
[0038] b. Remove iron and aluminum from the solution con...
Embodiment 3
[0042] Such as figure 1 As shown, get the nickel cobalt hydroxide raw material obtained by recovering the nickel cobalt raw material intermediate, take 1kg nickel cobalt hydroxide material as an example, in the nickel cobalt hydroxide material after testing: the content of Co is 5.5%, and the content of Ni is 37% , the content of Fe is 0.6%, the content of Al is 0.12%, the content of Ca is 0.5%, and the content of Mg is 0.8%. The above-mentioned nickel cobalt hydroxide raw material is processed as follows:
[0043] a. The above-mentioned nickel cobalt hydroxide raw material and water are made into a slurry according to the solid-to-liquid ratio of 1:10, then the pH of the solution is adjusted to 1.0 by adding sulfuric acid, the reaction temperature is controlled at 68°C, and the reaction time is 0.8h, and the nickel cobalt hydroxide The raw materials are dissolved into a solution containing nickel and cobalt sulfate.
[0044] b. Remove iron and aluminum from the solution con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com