A kind of drug combination containing plinabulin and application thereof
A drug and drug resistance technology, applied in the field of drug combinations containing plinabulin, can solve the problems of long-term malignant tumors, poor selectivity of tumor cells, drug resistance or drug resistance, etc., to achieve convenient drug administration and improve The effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] When the combination of Plinabulin and Tetrandrine is used in human treatment, the dosage of the two drugs is determined according to the phase II clinical data or instructions. The specific situation is: according to the Phase II clinical data of Plinabulin, Plinabulin The daily recommended dosage of Nabuline is 32-48mg, continuous medication for 4 weeks, 2 weeks of drug withdrawal, 6 weeks as a course of treatment. According to the instruction manual of tetrandrine, the daily recommended dosage of tetrandrine is 200 mg, administered twice a week for 2 consecutive weeks, and stopped for 1 week, repeating this twice, 6 weeks as a course of treatment. According to the average adult body weight of 60kg, the daily recommended dosage of Plinabulin is converted into clinical daily dosage per kilogram of body weight, and the daily dosage of Plinabulin is: 0.53-0.8mg / kg body weight / day. According to the average adult body weight of 60kg, the daily recommended dosage of tetrand...
experiment example 1
[0045] Experimental Example 1 Cell Experiment
[0046] 1. Cell selection and active culture
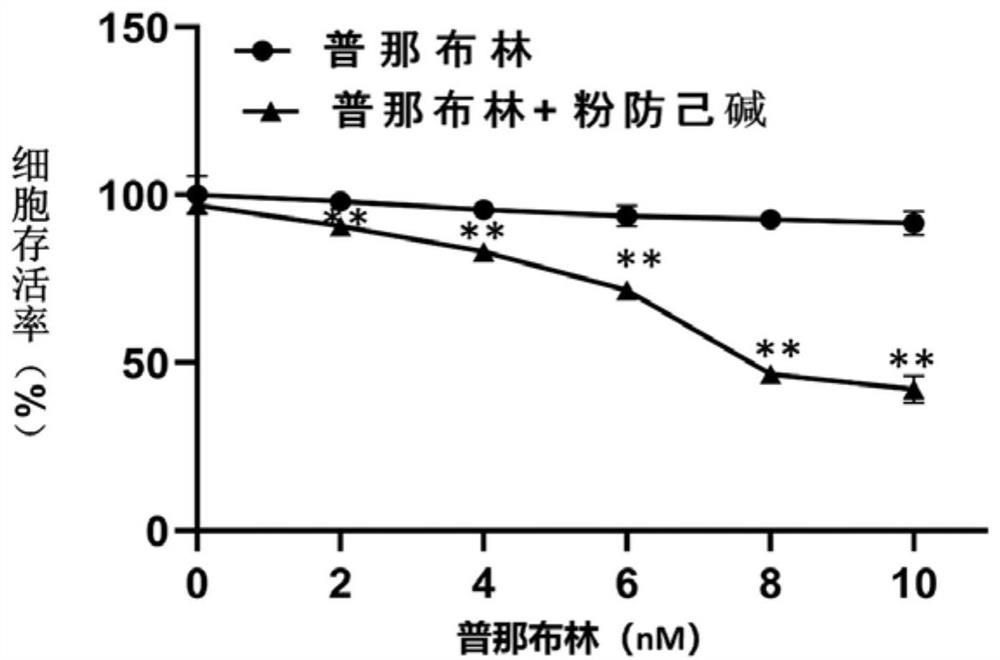
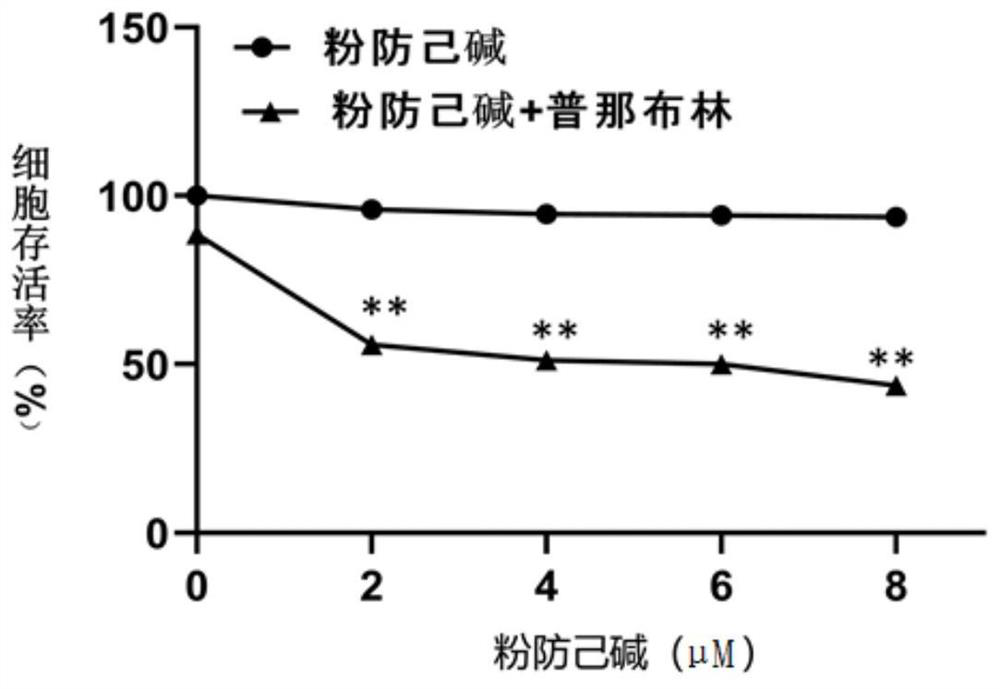
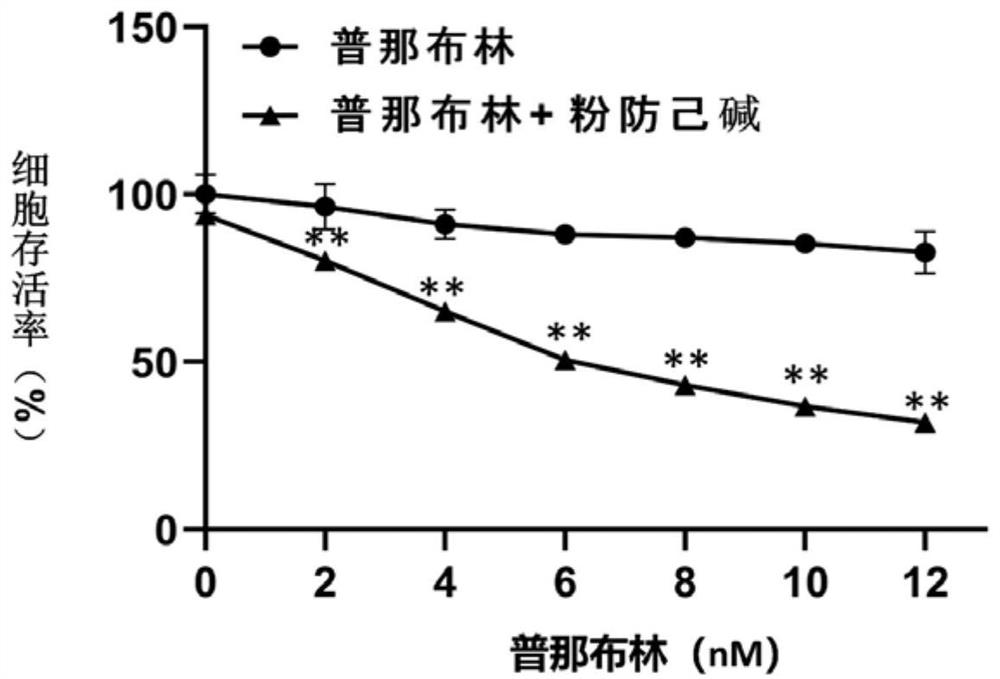
[0047] The experiments selected vincristine-resistant human gastric cancer cells SGC-7901 / VCR, human gastric cancer cells SGC-7901, human breast cancer cells MDA-MB-231, doxorubicin-resistant human chronic myelogenous leukemia cells K-562 / ADR, human Histiocytic lymphoma cells U-937. Vincristine-resistant human gastric cancer cells SGC-7901 / VCR, human gastric cancer cells SGC-7901, and human breast cancer cells MDA-MB-231 were cultured in DMEM+10% FBS complete medium. Human histiocytic lymphoma cells U-937 were cultured in 1640+10% FBS complete medium. Doxorubicin-resistant human chronic myeloid leukemia cells K-562 / ADR were cultured in IMDM+10% FBS complete medium. The above cells were kept at 37°C with 5% CO 2 , cultured in a cell incubator with saturated humidity.
[0048] 2. Experimental method
[0049] After each tumor cell line is in a stable state, spread the cells in a 96...
experiment example 2
[0079] Experimental example 2 Nude mouse transplantation tumor experiment
[0080] In the course of this experiment, the injection dose was based on the US Food and Drug Administration (FDA) "Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers" (Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers) ), the injection dose conversion ratio of human body and nude mice (ie, nude mice in experimental verification) required to achieve the same biological effect is calculated as 1:12.3. Based on this conversion ratio, the dosage of the nude mice used in the experiment was converted by comparing the dosage of the human body. Data were analyzed with Graphpad Prism 8.0 software, P<0.05 was considered significant difference (*P<0.05; **P<0.01; ***P<0.001).
[0081] In each embodiment, the dosage of tetrandrine is: 40.96mg / kg (3.33mg / kg×12.3) according to the body weight of nude mice, and the dosage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com