High-purity hemodialysis concentrated solution and preparation process thereof
A technology of hemodialysis and preparation process, which is applied in the field of high-purity hemodialysis concentrate preparation process, hemodialysis concentrate preparation process, and high-purity hemodialysis concentrate, which can solve the problem of low efficiency, affecting blood calcium, and the inability to adjust the pH balance. issues such as getting the right adjustments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the present embodiment, the raw material, the concentration of each component is: calcium chlorate 5g / L, citric acid 30g / L, heparin 5g / L, calcium chloride 10g / L, potassium chloride 10g / L, magnesium chloride 5g / L, sodium chloride 50g / L, glucose 5-10 g / l, iron citrate 450 mg / L, sodium gluconate iron 1500 mg / L and osmotic pressure regulating electrolyte.
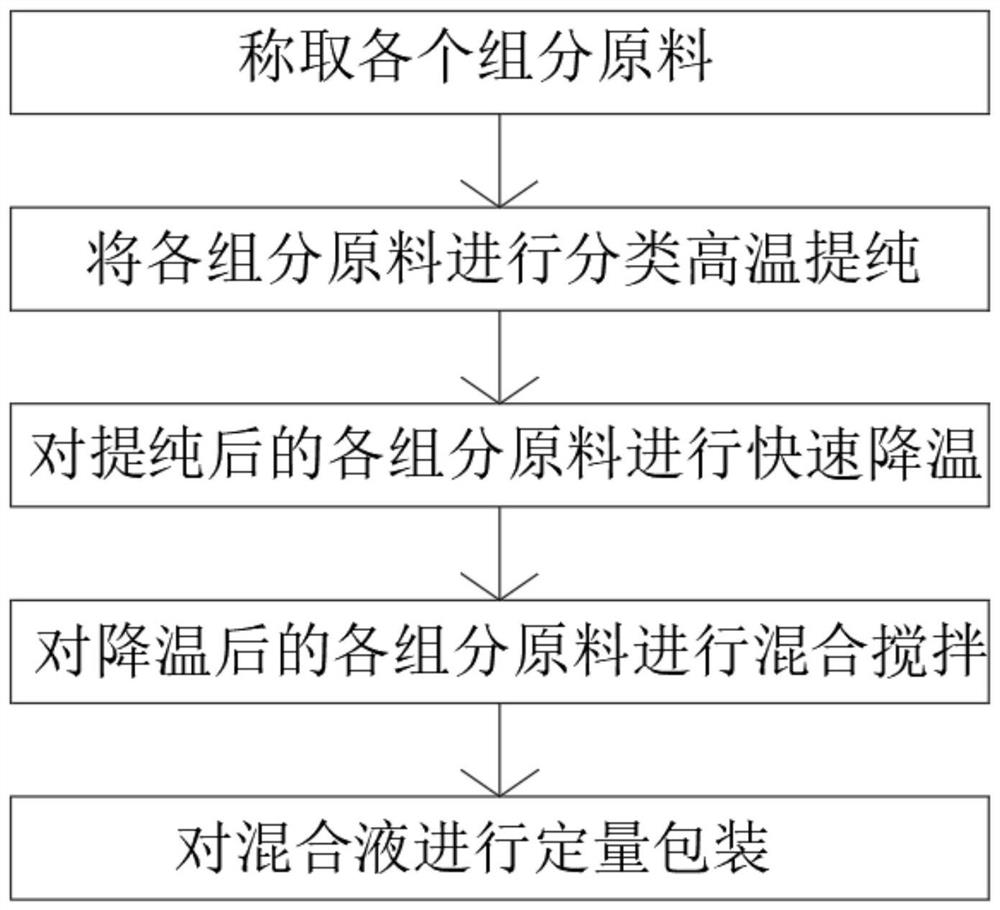
[0030] Based on the above raw material concentration, a high-purity hemodialysis concentrate is prepared, including the following steps:
[0031] Step 1: Take the calcium chlorate 10g / L, citric acid 60 g / L, heparin 25 g / L, calcium chloride 20g / L, potassium chloride, 10 g / L, sodium chloride 10g / L, sodium chloride, 100g / L, glucose 10g / L, iron citrate 950mg / l and sodium gluconate 3000mg / L;
[0032] Step 2: Put the individual components of the quantity into the purified box and placed in the purified box;
[0033] Step 3: Perform high temperature continuous heating 2-4H temperature main...
Embodiment 2
[0040] In the present embodiment, the raw material is taken: the concentration of each component is: calcium chlorate 10g / L, citric acid 60 g / L, heparin 25 g / L, calcium chloride 20g / L, potassium chloride 20g / L, magnesium chloride 10g / L, sodium chloride 100g / L, glucose 10g / L, citrate 950 mg / L, sodium gluconate 3000 mg / L and osmotic pressure regulating electrolyte.
[0041] Based on the above raw materials, the production step of hemodialysis concentrate is the same as that of the examples, and will not be explained herein.
Embodiment 3
[0043] In the present embodiment, the raw material: the concentration of each component is: calcium chlorate 7.5 g / L, citric acid 45 g / L, heparin 15g / L, calcium chloride 15g / L, magnesium chloride, magnesium chloride, magnesium chloride, magnesium chloride, magnesium chloride 7.5 g / L, sodium chloride 75 g / L, glucose 7.5 g / L, citrate 700 mg / L, gluconate 2250 mg / L and osmotic pressure regulated electrolyte.
[0044] Based on the above raw materials, the production step of hemodialysis concentrate is the same as that of the examples, and will not be explained herein.
[0045] Comparative Examples 1, II, two, from the preparation of high purity hemodialysis concentrate, the effect of the secondary hydrogen carbonate hemodialysis is the most reasonable, so the content of the blood dialysate is the most reasonable. Calcium glorate 10g / L, citric acid 60g / L, heparin 25g / L, calcium chloride 20g / L, potassium chloride 20g / L, sodium chloride 100g / L, glucose 10g / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com