Application of taurolidine in virus resistance
A technology of taurolidine and viruses, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, respiratory diseases, etc., can solve problems such as no research reports on the application of antiviral activity, and achieve significant protective effects, The effect of expanding the scope of efficacy and prolonging the survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
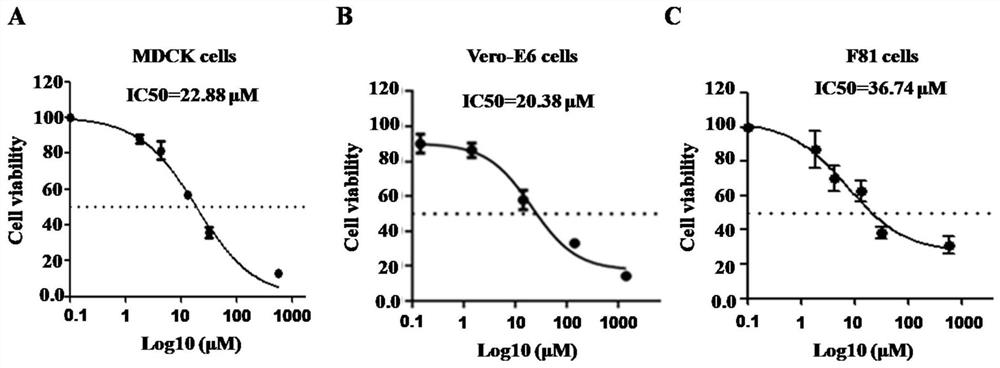
[0059] Example 1 The effect of taurolidine on cell safety
[0060] MDCK, Vero-E6, and F81 cells were stored in liquid nitrogen, taken out and recovered, and passed on for three consecutive generations. After the cells grew well, they were used for experimental research. MDCK, Vero-E6, and F81 cells were seeded in 96-well plates, and the number of cells in each well was 1 × 10. 5 , at 37°C, 5% CO 2 Incubate overnight in a constant temperature cell incubator. When the cell density is 60-70%, add 2% FBS DMEM containing different concentrations of taurolidine (initial concentration 4mg / ml, with 10-fold gradient to dilute the drug to be tested, a total of 10 concentrations) in 2% FBS DMEM. 100 μL / well of solution, and 4 replicate wells were measured for each concentration. A blank cell control and a PBS control were also set up, and 4 repetitions were performed, and the culture was continued, and the cell status was observed under a microscope every day. On the second day of do...
Embodiment 2
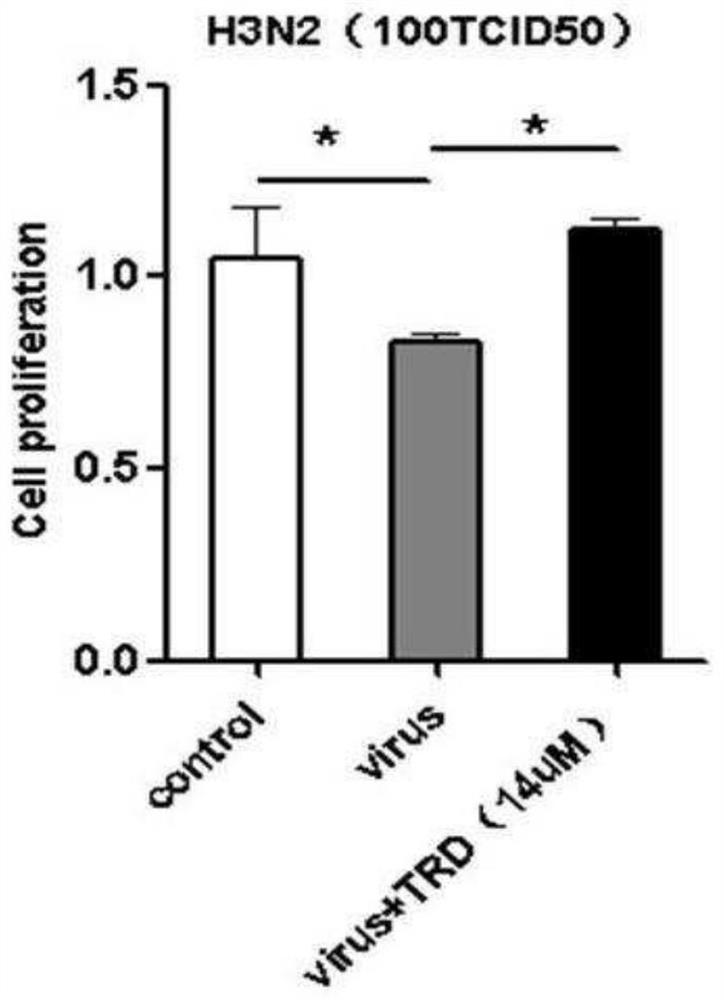
[0062] Embodiment 2 Taurolidine inhibits the detection result of influenza virus infectivity
[0063] The MDCK cells were stored in liquid nitrogen, taken out and recovered, and passed on for three consecutive generations. After the cells grew well, they were used for experimental research. The preserved H3N2 influenza virus strain was slowly thawed on ice, inoculated into a monolayer of MDCK cells (less than 24 hours), and cultured for 72-96 hours, and the virus liquid was harvested according to the cytopathic state. and determine its content, with TCID 50 / 100μl is the calculation unit.
[0064]The drug is mixed with the H3N2 subtype influenza virus liquid obtained above and then inserted into the cells. The well-growing MDCK cells were digested with trypsin to calculate the cell content, and seeded in a 96-well plate, the number of cells per well was 1×10 5 . Drug effect studies were performed within 12 hours of seeding and when the cells were in a monolayer state. Ino...
Embodiment 3
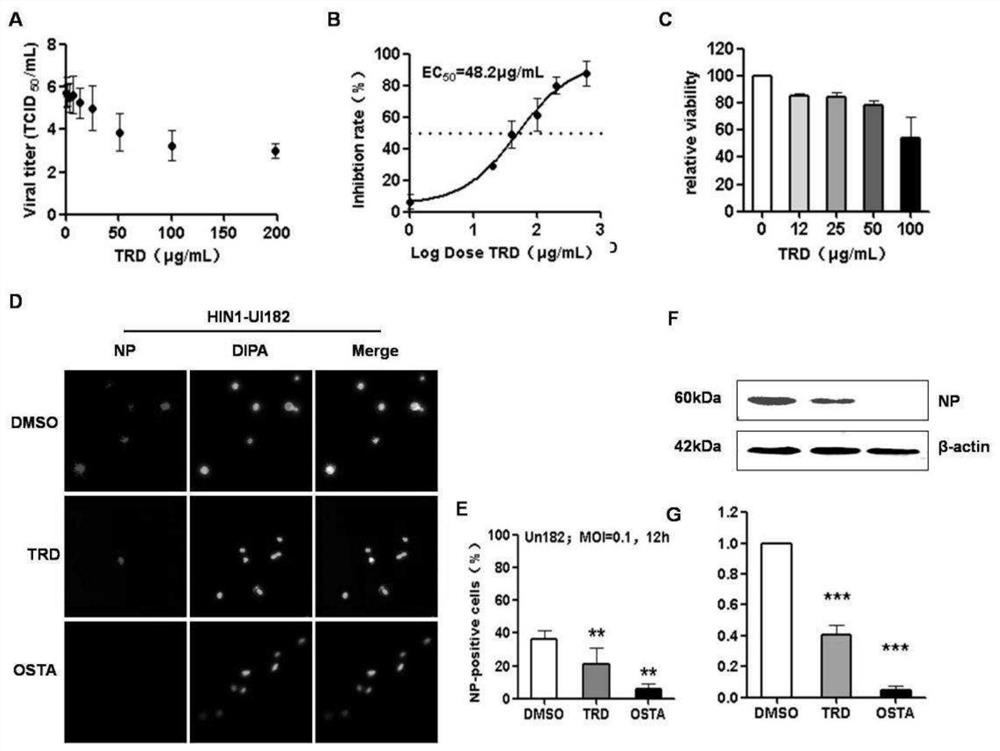
[0067] Embodiment 3 Taurolidine inhibits the propagation of influenza virus in cells
[0068] 1. Experimental materials
[0069] 1.1 Cells: human lung cancer cell A549 and African green monkey kidney cell MDCK, from the Virology Laboratory of the Military Veterinary Research Institute;
[0070] 1.2 Virus strains: H1N1-UI182, H1N1-PR8, H3N2 and H5N1 viruses, from the Virology Laboratory of the Military Veterinary Research Institute;
[0071] 1.3 Reagents: DMEM, 0.25% trypsin, FBS, PBS (pH=7.0);
[0072] 1.4 Instrument consumables: pipette and supporting tips, 1.5mL centrifuge tube, ice box, ice maker, biological safety cabinet, carbon dioxide incubator.
[0073] 2. Experimental method
[0074] 2.1 Cell culture: Resuscitate A549 and MDCK, pass three generations continuously, and use for experimental research after the cells grow well;
[0075] 2.2 Virus culture: Put the preserved virus liquid on ice to slowly melt and inoculate it in a single layer of MDCK (no more than 24 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com