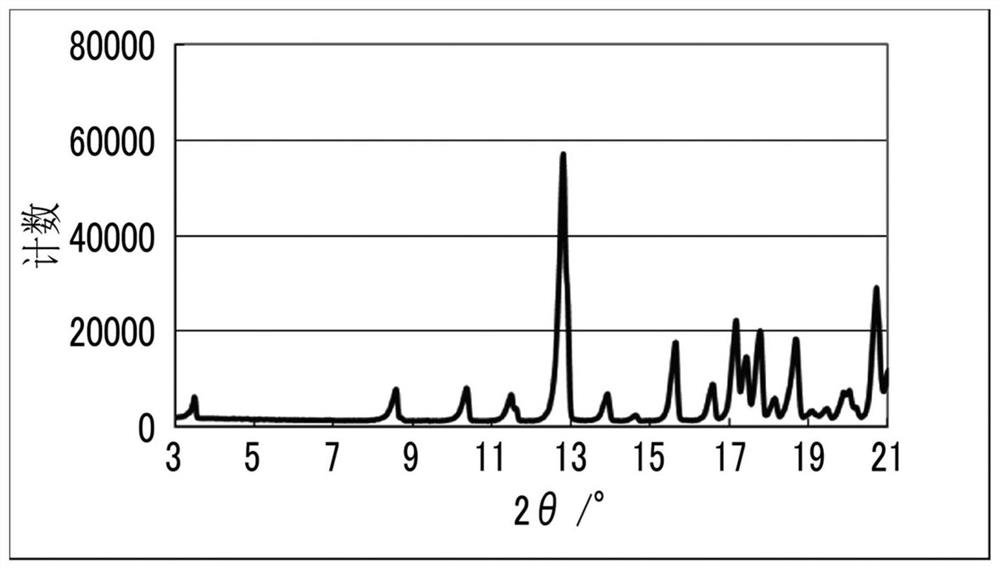
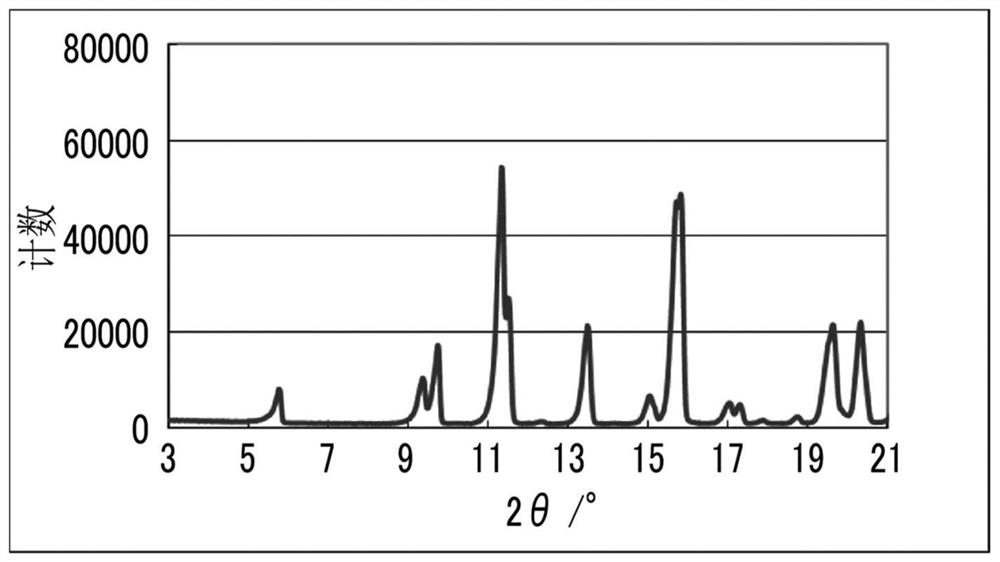
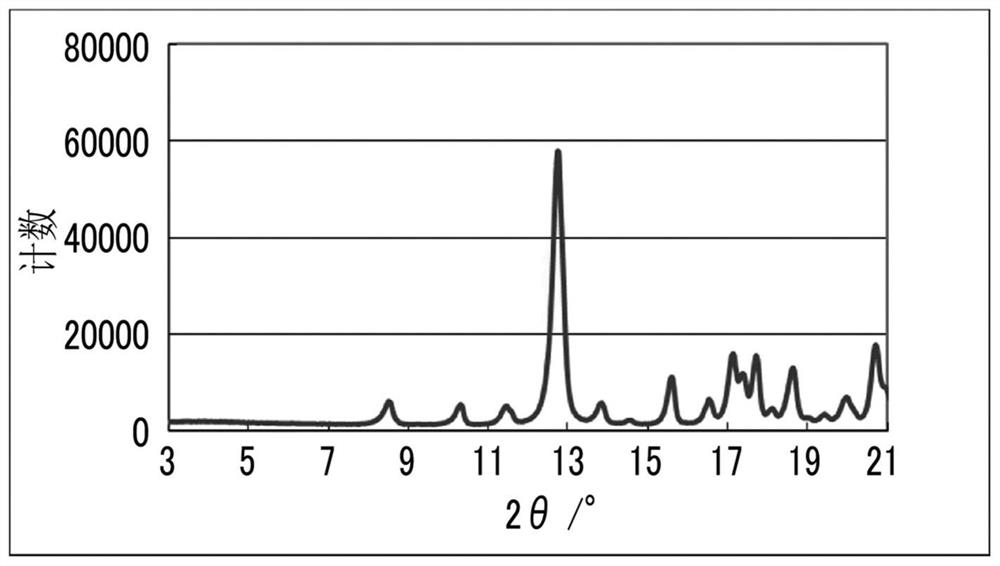
Solvate and method for producing solvate
A solvate and solvent technology, applied in the field of manufacturing solvates and solvates, can solve problems such as chromatic aberration, and achieve the effects of high-yield manufacturing and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0146] Hereinafter, an Example and a comparative example are given, and the characteristics of this invention are demonstrated more concretely. Unless departing from the gist of the present invention, the materials, usage amounts, ratios, processing contents, processing procedures, and the like shown in the following examples can be appropriately changed. Therefore, the scope of the present invention should not be limitedly interpreted by the specific examples shown below.
[0147]
Synthetic example 1
[0148] Synthesis example 1 (compound c3):
[0149] 150 mL of toluene, 125 mL of ethanol, and 100 mL of acetic acid were added to 26.6 g of 4,5-dimethyl-1,2-phenylenediamine and 35.6 g of ninhydrin, and reacted at 70° C. for 3 hours. After cooling the reaction solution to room temperature, the precipitated crystals were collected by filtration, washed with ethanol, and dried to obtain 47 g of intermediate c2A.
[0150] 1 H-NMR (300MHz, CDCl 3 ): δ2.49ppm(s,3H), 2.51ppm(s,3H), 7.52-7.58ppm(t,1H), 7.71-7.76ppm(t,1H), 7.85-7.95ppm(m,3H), 8.02 -8.08ppm(d,1H)
[0151] 22 g of intermediate c2A and 32 g of phenol were dissolved in 20 mL of methanesulfonic acid and 20 mL of acetonitrile. The reaction solution was heated, and 0.3 mL of 3-mercaptopropionic acid was added dropwise while maintaining 90°C. After stirring for 3 hours, 200 mL of acetonitrile and 100 mL of water were added, and the reaction solution was stirred at 40° C. for 2 hours. The precipitated crystals were collec...
Synthetic example 2
[0153] Synthesis example 2 (compound c1):
[0154] Except having changed 4, 5- dimethyl- 1, 2- phenylenediamine into o-phenylenediamine, 28 g of compound c1 were obtained by the method similar to synthesis|combination of compound c3.
[0155] 1 H-NMR (300MHz, DMSO-d 6 ): δ6.61-6.68ppm(d,4H), 6.95-7.01ppm(d,4H), 7.50-7.70ppm(m,3H), 7.72-7.90ppm(m,2H), 8.00-8.08ppm(d ,1H), 8.12-8.25ppm(m,2H), 9.41ppm(bs,2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com