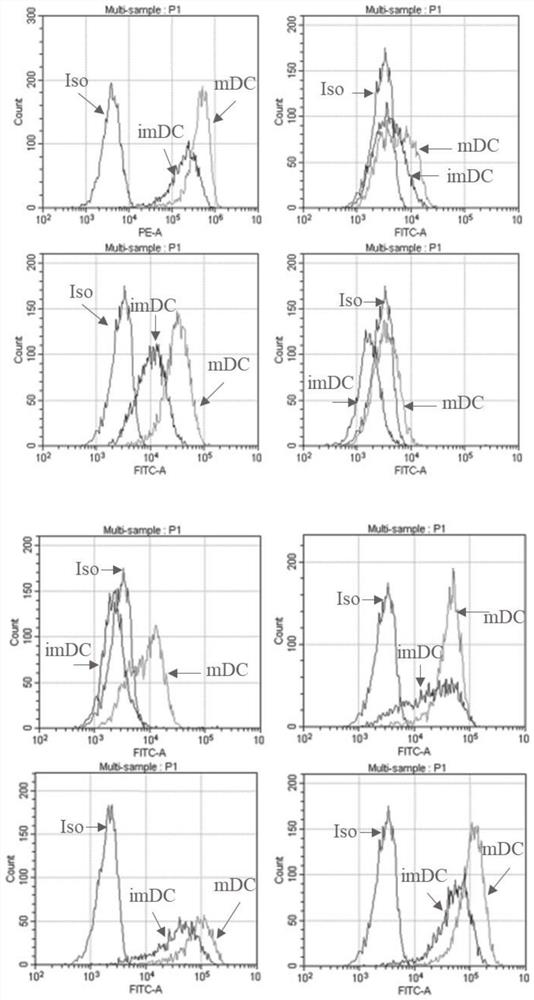
EBV (Epstein-Barr Virus) composite antigen, dendritic cell vaccine and application thereof
A technology of dendritic cells and complex antigens, applied in the field of biomedicine, can solve the problems of lack of anti-tumor immune response, killing of infected cells, immune escape, etc., and achieve good clinical application prospects, inhibit the evolution of cancer cells, and reduce immune resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Peripheral Blood Mononuclear Cells PBMC Isolation
[0049] This embodiment is based on the difference in the density of each cell component in peripheral blood (peripheral blood mainly contains cells such as platelets, mononuclear cells, granulocytes, and red blood cells: the density of platelets is 1.030-1.035kg / m 3 , the mononuclear cell density is 1.075-1.090kg / m 3 , the granulocyte density is 1.092kg / m 3 , red blood cell density is 1.093kg / m 3 ), added in peripheral blood samples Paque Plus (GE Healthcare) solution (density 1.075-1.089kg / m 3 ), performing density gradient centrifugation to stratify different cell components, and can quickly separate mononuclear cells from human peripheral blood.
[0050] (1) Collect peripheral blood from the veins of EBV-infected patients, use centrifuge tubes of corresponding specifications, and use pipettes to add 4.5mL of Paque Plus solution.
[0051] (2) Use a pipette to draw blood samples and slowly inject th...
Embodiment 2
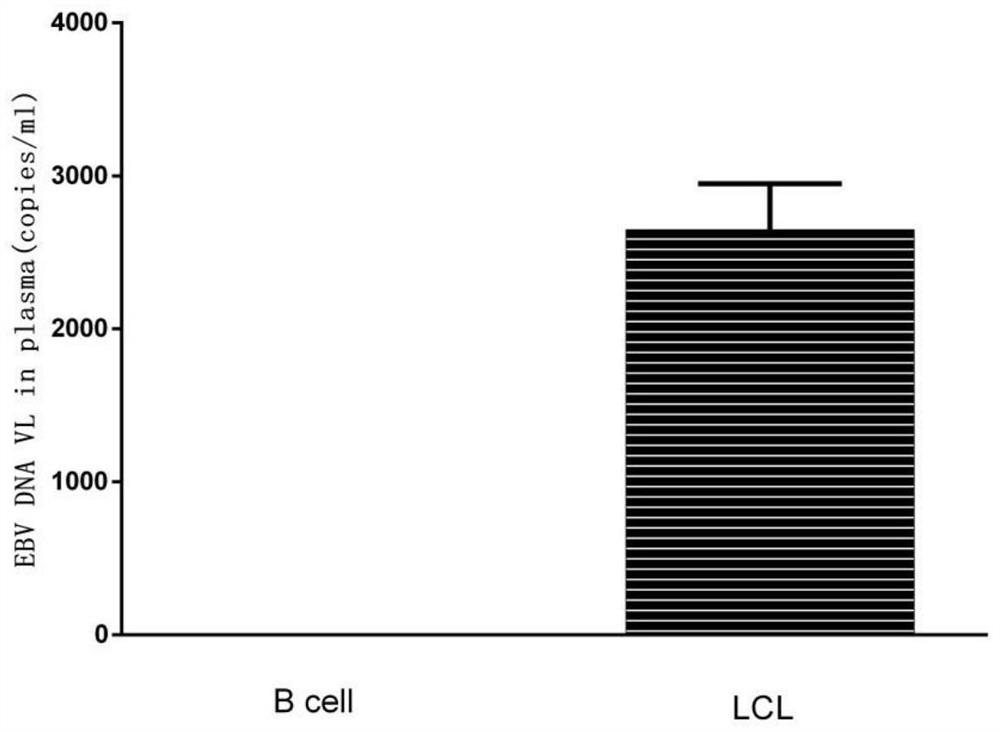
[0059] Embodiment 2: Immortalization human B lymphocyte line LCLs of construction Epstein-Barr virus strain infection
[0060] (1) Transfer 10 mL of B95-8 cell supernatant to a centrifuge tube, centrifuge at 2000 rpm for 15 min, and filter the supernatant.
[0061] (2) Resuspend the PBMC prepared in Example 1 in 2 mL of RPMI1640 / 10% FBS medium.
[0062] (3) Aspirate 10 μL of cell solution, add 90 μL RPMI / 10% FBS to dilute 10 times, and perform cell counting under a microscope. According to the counting results, calculate the required volume of B95-8 supernatant, where every 1×10 6 One PBMC cell corresponds to 1 mL of B95-8 supernatant.
[0063] (4) PBMC cells were collected, centrifuged at 1000 rpm for 5 min, and the PBMC supernatant was discarded.
[0064] (5) According to the cell counting results, add an appropriate amount of B95-8 cell supernatant to resuspend PBMC cells, so that the concentration of PBMC cells in the cell solution is 0.5×10 6 / 500 μL.
[0065] (6) Pr...
Embodiment 3
[0079] Embodiment 3: the preparation of EBV cell lysate
[0080]The repeated freezing and thawing method is a commonly used mechanical lysis method, usually consisting of two parts (freezing and thawing). The principle is that the formation of ice particles in the cells and the increase in the salt concentration of the remaining cell fluid cause swelling, which breaks the cell structure and causes cell death, but retains the immunogenicity of the cells. Freezing is usually carried out in liquid nitrogen or on ice at -20°C, and thawing can be carried out in a water bath at 37°C, 50°C, 65°C or 100°C, which is milder than chemical lysis.
[0081] (1) Set the water bath temperature to 37°C in advance.
[0082] (2) Collect immortalized human B lymphocyte line LCLs or EBV-positive infected cells (such as C666-1 cells, HNE1, CCL85 or other EBV-infected T cells, NK cells or B cells) (at least 3×10 7 ), centrifuged at 700 g for 5 min at room temperature to harvest the cells.
[0083...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com