A method for high-throughput screening of single-domain antibodies using ispla and its application
A single-domain antibody, high-throughput technology, applied in the biological field, can solve the problems of lack of high affinity, high specificity, undiscovered patent publications, low sdAb success rate, etc., to achieve easy mass expression and purification, low cost, The effect of reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The embodiments of the present invention are described in detail below. It should be noted that the embodiments are descriptive, not restrictive, and cannot limit the protection scope of the present invention.
[0030] The raw materials used in the present invention are conventional commercial products unless otherwise specified; the methods used in the present invention are conventional methods in the art unless otherwise specified.
[0031] A method for high-throughput screening of single-domain antibodies using isPLA, the steps are as follows:
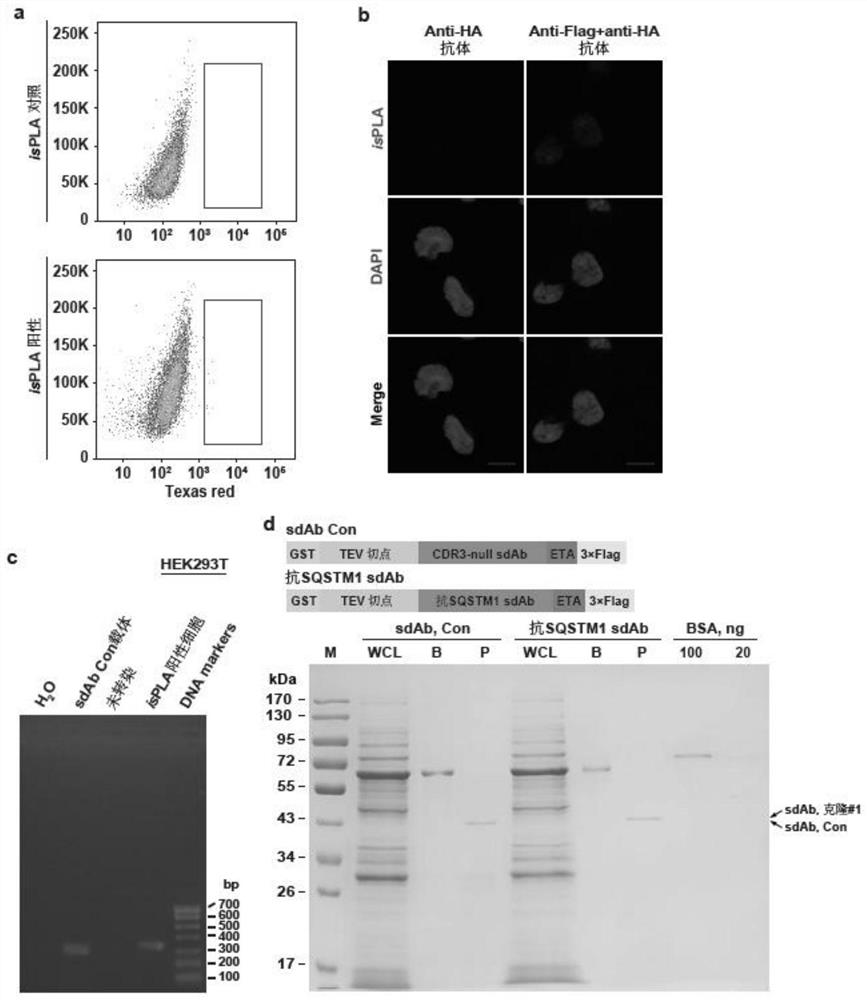
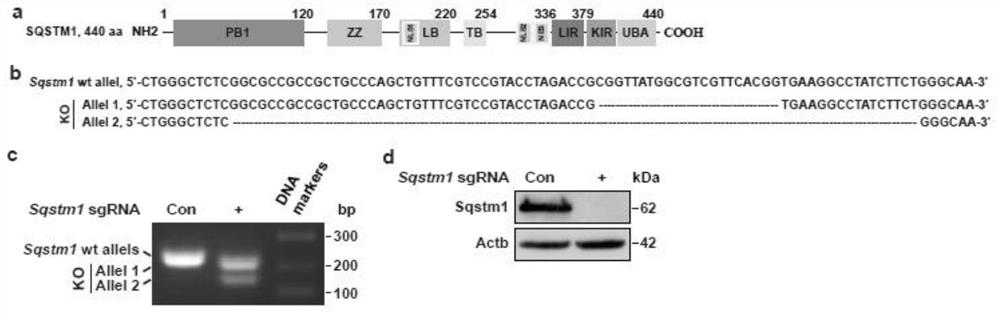
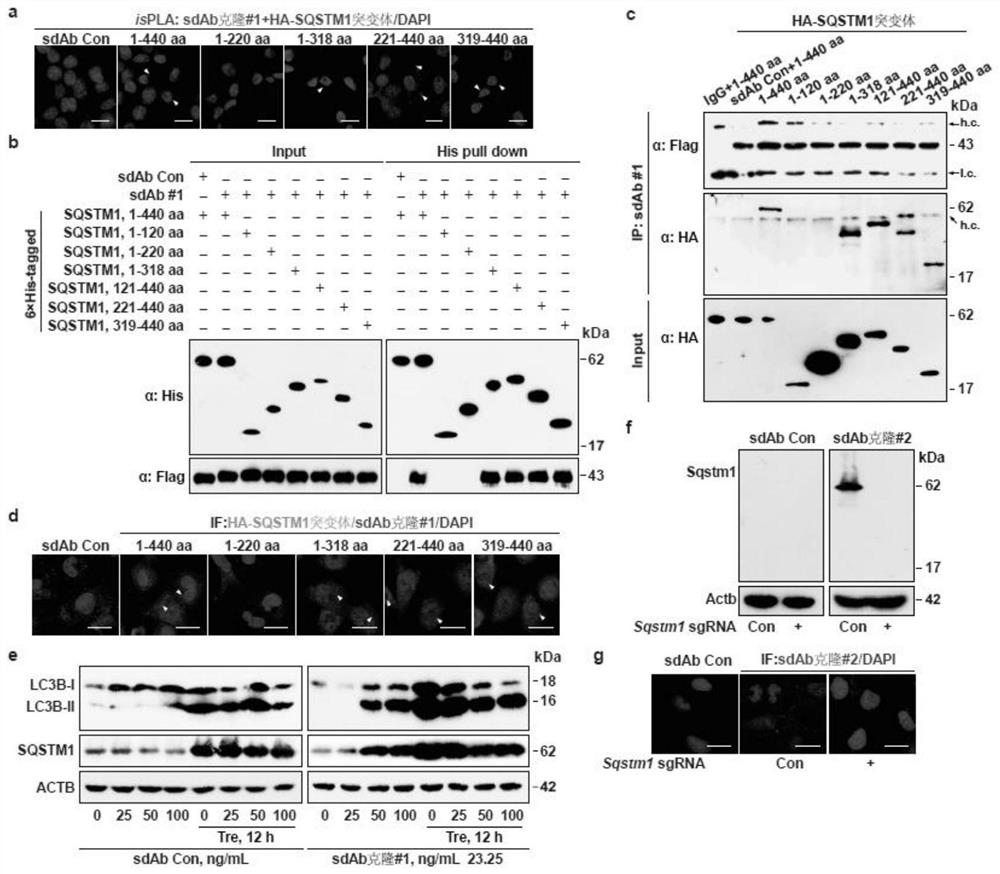
[0032] First, a sdAb library containing 21 random amino acid sequences in the CDR3 region was constructed, and a 3×Flag tag was fused to the C-terminus of the sequence; HEK293T cells were co-transfected with the library plasmid and the HA-tagged SQSTM1 expression plasmid. Cells were fixed for isPLA; cells with positive red fluorescent signals were then sorted; the plasmids in these cells contained DNA sequences encoding sdAbs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com