Detection method of aav protein coat
A detection method and shell technology, applied in the direction of immunoglobulin, chemical instruments and methods, antiviral immunoglobulin, etc., can solve the problems of inaccurate quantitative detection and achieve high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Production of Antigens
[0053] The intact hollow AAV protein capsid (AAVXL32.1) was produced as antigen by transfecting HEK293 cells with the two plasmids. AAV rep / cap plasmid: phelper: polyethyleneimine (PEI) = 1:1:2, mixed in Opti-MEM and left to stand for 12 min, the transfection solution was added to the suspended HEK293 cells. After 24 h in the carbon dioxide incubator, the old medium was discarded and new medium was added. After culturing for 24 hours, the supernatant and cells were collected. Add 2mM MgCl 2 , for ultrasonication. After 4 min of disruption at 300W, the cells were disrupted. Add 50 U / mL of Benzonase enzyme, incubate at 37 °C for 1 h, and centrifuge at 8000 × g for 15 min to remove cell debris. The supernatant was filtered sequentially using 0.8 μm and 0.45 μm filters.
[0054] Heparin-Sepharose HP (Heparin-Sepharose HP) was used for capture, and the heparin column was first equilibrated with equilibration solution (20mM Tris, 20...
Embodiment 2
[0055] Example 2: Acquisition of Hybridoma Cells
[0056] immunized animals
[0057] 6-8 week-old female Balb / c mice were immunized with the antigen obtained in Example 1, and the mice were made to generate sensitized B lymphocytes. Mice were killed by enucleation and exsanguination, and the spleen was taken out aseptically, squeezed and ground in a plate to prepare a spleen cell suspension.
[0058] Generation of hybridoma cells
[0059] Syngeneic myeloma cells were mixed 1:1 with mouse splenocytes and the fusogenic agent polyethylene glycol was added. Under the action of polyethylene glycol, B lymphocytes fuse with myeloma cells to form hybridoma cells. After fusion of B lymphocytes and myeloma cells, the following five cell types are generated: unfused spleen cells, unfused myeloma cells, spleen cell-spleen cell fusions, myeloma cells-myeloma cell fusions, and spleen cells-bone marrow Tumor cell hybrids (hybridoma cells). Then, using hybridoma cell screening tech...
Embodiment 3
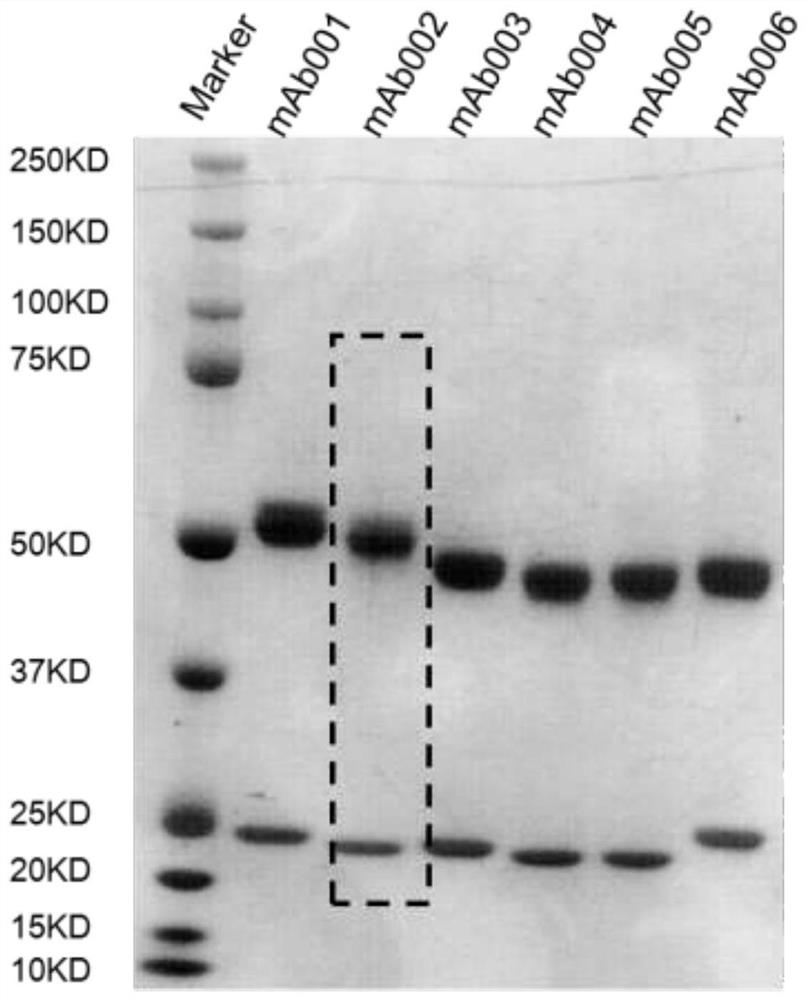
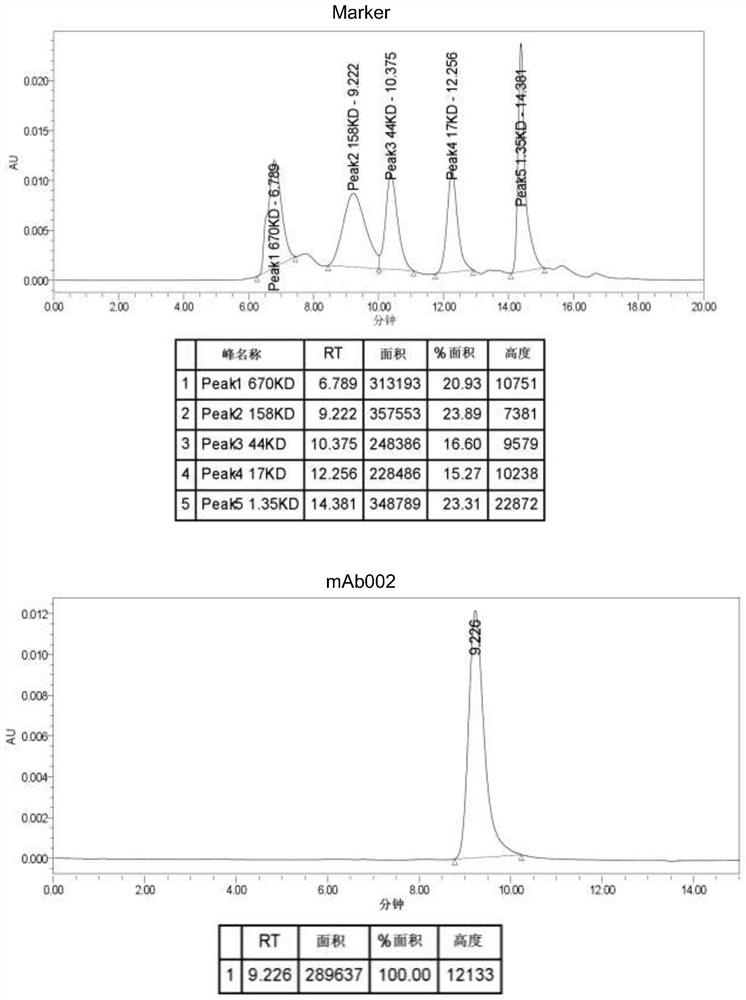
[0062] Example 3: Production and Purification of Monoclonal Antibodies
[0063] Cells were harvested from the flasks and the number of viable cells was determined. If viability is above 80%, seed cells into roller bottles prefilled with 200 mL of antibody production medium (Hybridoma-SFM + 2.5% FBS (low IgG)) at an initial cell density of 0.25 x 10 5 cells / mL to 0.5 x 10 5cells / mL. After inoculation, incubate at 300 r / h in a roller bottle incubator without CO. 2 The cells were cultured at 37 °C for 14-16 days, and the cell culture supernatant was collected. Then, the cell suspension was transferred to a 350 ml centrifuge bottle and centrifuged at 3220 x g for 15 min at 4 °C, followed by filtration with a 0.45 μm filter to remove cells and cell debris.
[0064] The cell culture supernatant was loaded onto a pre-equilibrated Protein A affinity column, which was then washed with 10 CV of equilibration buffer (1x PBS, pH 7.2) until the OD of the flow-through sample became ze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com