Oxidation precipitation method for boron
A hydrogen peroxide and calcium chloride technology, applied in chemical instruments and methods, flocculation/sedimentation water/sewage treatment, water/sewage multi-stage treatment, etc., can solve the problems of complex boron removal process and low boron removal efficiency, and achieve technological Simple, improve boron removal efficiency, no secondary pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A boron oxidation precipitation method, adding hydrogen peroxide to the boron-containing waste liquid to react for a period of time, then adjusting the pH of the boron waste liquid to the range of 12-13, adding calcium chloride to react for a period of time, and then adding sulfuric acid to the boron waste liquid Aluminum, after another 10 minutes of reaction, drop the PAM reagent, and let the flocs settle.
[0026] Wherein, the reaction process is carried out in a container, and the container is placed on a magnetic stirrer, so that the magnetic stirrer is continuously stirred during the reaction process.
Embodiment 2
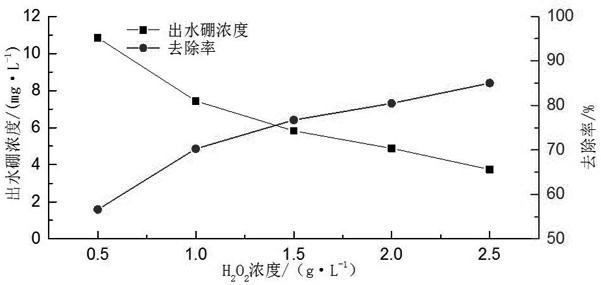
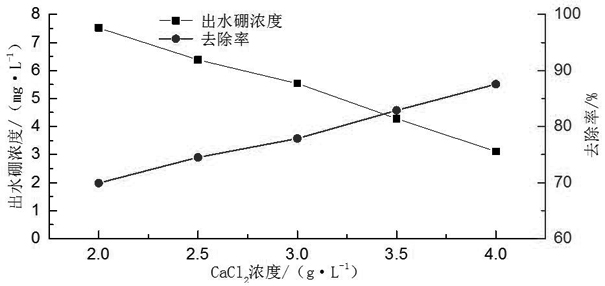
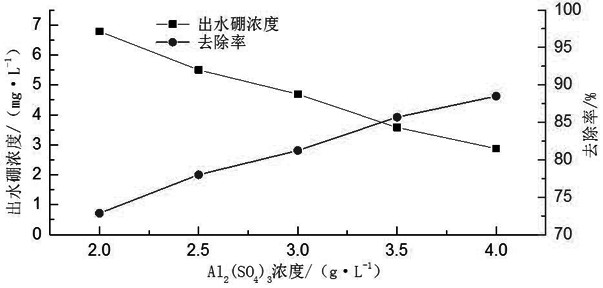
[0028] A boron oxidation precipitation method, adding 0.5-2.5g / L hydrogen peroxide to the boron-containing waste liquid to react for 30-90min, then adjusting the pH of the boron waste liquid to the range of 12-13, and then adding calcium chloride to make chlorine The concentration of calcium chloride in the boron waste liquid is 2.0-4.0g / L, after reacting for 20-60min, add aluminum sulfate to the boron waste liquid, so that the concentration of aluminum sulfate in the boron waste liquid is 2.0-4.0g / L, and then After 10 minutes of reaction, 3 drops of 0.2% PAM reagent were added dropwise, and the flocs were allowed to settle.
Embodiment 3
[0030] A boron oxidation precipitation method, adding 1.5g / L hydrogen peroxide to the boron-containing waste liquid to react for 30 minutes, then adjusting the pH of the boron waste liquid to the range of 12-13, and then adding calcium chloride to make the calcium chloride in the boron The concentration in the waste liquid is 3.5g / L. Add aluminum sulfate to the boron waste liquid after reacting for 30 minutes, so that the concentration of aluminum sulfate in the boron waste liquid is 3.0g / L. After another ten minutes of reaction, add 3 drops of 0.2% PAM reagent, let the flocs settle.
[0031] Using this scheme to remove boron from wastewater can increase the boron removal efficiency from the original 44.5% to 88.4%, which greatly improves the boron removal efficiency. Requirements, and this scheme is simple in process, low in cost, and does not produce secondary pollution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com