An active drug for pediatric asthma
A technology for children with asthma and drugs, which is applied in the field of acid addition salt pharmaceutical compositions, and can solve problems such as non-synergistic interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Safety Experiment: Cardiovascular Interaction of Compound A and Compound B
[0027] The post-administration peak changes in blood pressure and heart rate of male rats were determined after compound A and compound B were administered alone or in combination. The study design adopts a random blind method, selects 12 healthy rats with an average body weight of 220g, and divides them into 3 groups, and each group of four rats in the 3 groups is given compound A (4 μg / kg) and compound B (8 μg / kg) respectively. , Compound A (4 μg / kg) + Compound B (8 μg / kg). The primary pharmacokinetic variables measured were systolic blood pressure, diastolic blood pressure, and heart rate using calculated mean blood pressure. No major interactions between the drugs were found through the tests.
Embodiment 2
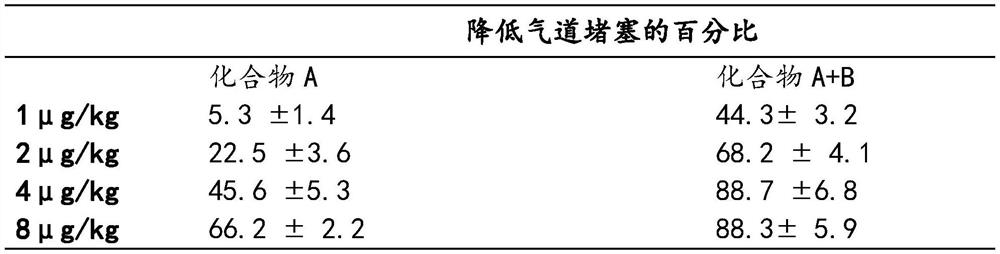
[0029] Synergistic Effect of Combination Administration of Compound A and Compound B on Reducing Airway Obstruction
[0030] The experiment induced airway obstruction by intravenous administration of bombesin (4 μg / ml) to guinea pigs. Different doses of compound A (1 μg / kg, 2 μg / kg, 4 μg / kg, 8 μg / kg) were administered intravenously alone, and compound A (1 μg / kg, 2 μg / kg, 4 μg / kg, 8 μg / kg) were administered separately Combined with compound B (8 μg / kg), the effect of compound B on reducing airway obstruction of compound A was investigated.
[0031]
[0032] This result provides further evidence that when Compound B can significantly enhance the bronchodilation activity of Compound A.
Embodiment 3
[0034] Synergistic effect of compounds A+B on tracheal rings
[0035] Methods: 200-300g male Wistar rats were sacrificed, the neck was dissected, the trachea was excised 1mm above the tracheal bifurcation and 1mm below the thyroid cartilage, and a 3mm thick trachea was cut in Krebs-Henseleit solution ventilated with 95% oxygen-5% carbon dioxide at room temperature. The rings are divided into 6 groups. Wash twice with Krebs-Henseleit solution aerated with 95% oxygen-5% carbon dioxide, pH 7.0±0.5, apply a 1 g load to each ring, and let stand for 60 minutes.
[0036] Three groups were selected, and 150 μmol / L acetylcholine was added to stimulate the trachea. When the trachea fully contracted, the elution returned to the baseline, and the experiment began after the trachea rested for 30 minutes. 150 μmol / L acetylcholine was stimulated again, and then three groups were added compound A (4 mg), compound B (8 mg) and compound A (4 mg) + compound B (8 mg) dissolved in DMSO respective...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com