Method for preparing cyclic carbonate through catalysis of sulfonated metal Salen and polyether ionic liquid binary system
A technology of cyclic carbonates and ionic liquids, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Reduced activity of binary catalysts, lengthy and complex synthesis routes, etc., to achieve long life and loss, high catalytic activity and selectivity, and low loss effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9
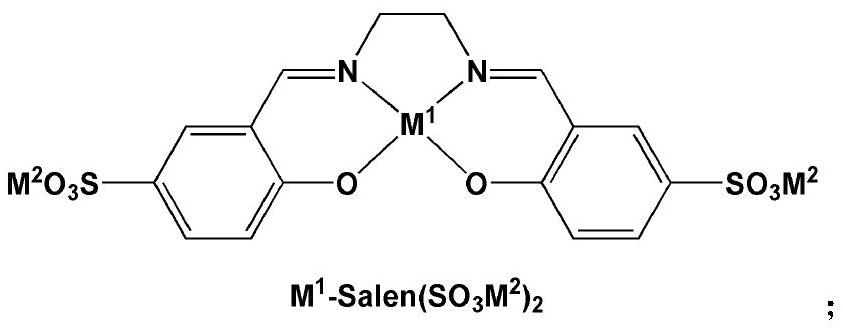
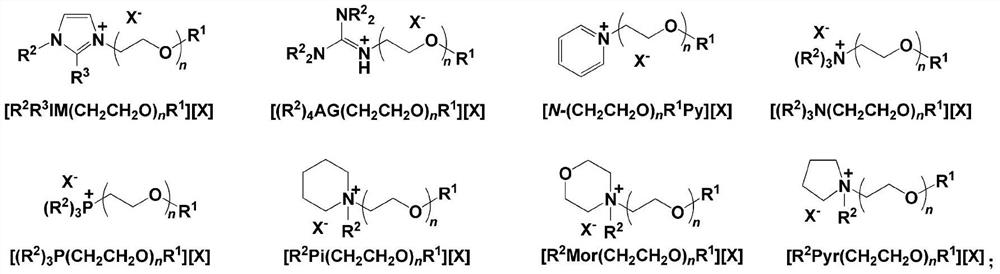
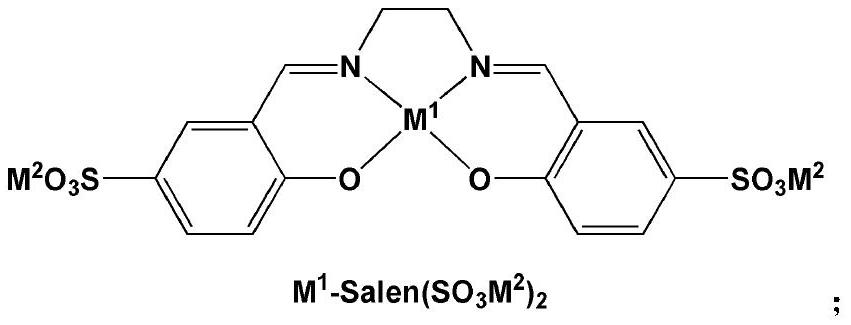
[0066] Zn-Salen (SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Me][Br] binary catalytic system (M 1 =Zn,M 2 =Na + , R 1 =Me,R 2 = Me,R 3 =H, n=16, X=Br) catalyzed CO 2 Cycloaddition reaction with styrene oxide (SO)
[0067] The binary catalyst was replaced by Zn-Salen(SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Me][Br], other operations are the same as in Comparative Example 7, SO conversion rate is 97.8%, cyclic carbonate selectivity is 97.3%, cyclic carbonate yield is 95.2%, and the TOF value when SO conversion rate reaches 50% is 1931h -1 .
[0068] Description: comparative example 9 and comparative example 7, Zn-Salen (SO 3 Na) 2 Substitution of Zn-Salen resulted in significantly improved catalytic activity.
Embodiment 10
[0070] Zn-Salen (SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Me][I] binary catalytic system (M 1 =Zn,M 2 =Na + , R 1 =Me,R 2 = Me,R 3 =H, n=16, X=I) Catalyzed CO 2 Cycloaddition reaction with styrene oxide (SO)
[0071] The binary catalyst was replaced by Zn-Salen(SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Me] [I], other operations are the same as Comparative Example 7, SO conversion rate 85.6%, cyclic carbonate selectivity 95.6%, cyclic carbonate yield 81.8%, the TOF value when SO conversion rate reaches 50% is 1030h -1 .
Embodiment 11
[0073] Zn-Salen (SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Ph][Br] binary catalytic system (M 1 =Zn,M 2 =Na + , R 1 =Me,R 2 = Ph, R 3 =H, n=16, X=Br) catalyzed CO 2 Cycloaddition reaction with styrene oxide (SO)
[0074] The binary catalyst was replaced by Zn-Salen(SO 3 Na) 2 / [MeIM(CH 2 CH 2 O) 16 Ph][Br], other operations are the same as in Comparative Example 7, the SO conversion rate is 95.8%, the cyclic carbonate selectivity is 95.9%, the cyclic carbonate yield is 91.9%, and the TOF value when the SO conversion rate reaches 50% is 1287h -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com