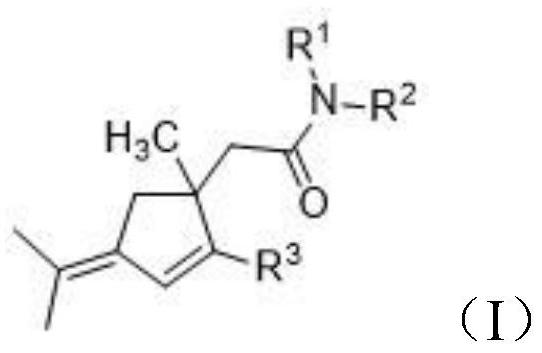
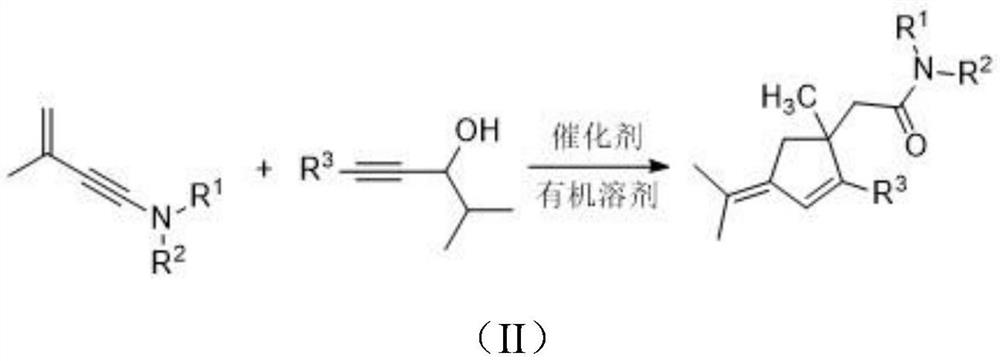
Penta-substituted 3-alkenylene cyclopentene derivative as well as synthesis method and application thereof
A technology for the synthesis of alkenylene cyclopentene, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., and can solve the problem of directly constructing 3-alkenylene Cyclopentene compounds, raw materials are not easy to obtain, and the environment is not friendly, etc., to achieve the effect of easy synthesis and modification, excellent yield, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 1-phenyl-5-methyl-5-(N-methyl-N-Tscarbamoylmethyl)-3-(2-propenylidene)cyclopentene (IA)
[0030]
[0031] Under nitrogen atmosphere, add 0.3mmol of 3-methyl-3-butene-1-ynamine compound, 0.36mmol of acetylenic alcohol, 3mL of 1,2-dichloroethane, PPh into a 10ml reaction tube 3 AuCl 0.03mmol, AgOTf 0.03mmol. React at 25°C for 6 hours to obtain the target product formula (IA), a light yellow oily liquid, with an isolation yield of 80%.
[0032] Product NMR data: 1H NMR (400MHz, CDCl 3 ):δ7.55(d,J=8.2Hz,2H),7.20-7.27(m,7H),6.39(s,1H),3.21(s,3H),3.07(d,J=17.1Hz,1H) ,2.74(d,J=17.0Hz,1H),2.72(d,J=15.7Hz,1H),2.48(d,J=16.2Hz,1H),2.40(s,3H),1.77(s,3H) ,1.67(s,3H),1.31(s,3H);13C NMR(100MHz,CDCl3):δ171.5,152.6,144.5,137.3,136.5,136.4,129.8,128.6,128.1,127.5,127.1,126.9,122.8,48.4 , 44.9, 44.3, 32.9, 26.9, 21.5, 21.2, 20.6.
[0033] High resolution mass spectrometry data: HRMS(ESI,m / z)calcd.for C 25 h 29 NNaO 3 S[M+Na]+calc.: 446.1766; found: 446.1759.
Embodiment 2
[0035] Synthesis of 1-(4-bromophenyl)-5-methyl-5-(N-methyl-N-Tscarbamoylmethyl)-3-(2-propenylidene)cyclopentene (IB)
[0036]
[0037] Under nitrogen atmosphere, add 0.3mmol of 3-methyl-3-butene-1-yneamine compound, 0.36mmol of acetylenic alcohol, 3mL of 1,2-dichloroethane, PPh into a 10ml reaction tube 3AuCl 0.03mmol, AgOTf 0.03mmol. React at 25°C for 5 hours to obtain the target product formula (IB), a pale yellow oily liquid with an isolated yield of 70%.
[0038] Product NMR data: 1H NMR (400MHz, CDCl 3 ): δ7.48(d, J=8.2Hz, 2H), 7.30(d, J=8.4Hz, 2H), 7.18(d, J=8.2Hz, 2H), 7.05(d, J=8.4Hz, 2H ),3.15(s,3H),2.95(d,J=17.2Hz,1H),2.68(d,J=17.2,2H),2.37-2.41(m,4H),1.71(s,3H),1.60( s,3H),1.22(s,3H);13C NMR(100MHz,CDCl3):δ171.3,151.0,144.7,136.4,136.2,136.2,131.2,129.9,129.2,129.1,127.0,123.7,120.8,48.3,44.8, 44.3, 32.9, 27.2, 21.6, 21.3, 20.7.
[0039] High resolution mass spectrometry data: HRMS(ESI,m / z)calcd.for C 25 h 28 BrNNaO 3 S[M+Na]+calc.: 524.0871; found: 524.0...
Embodiment 3
[0041] Synthesis of 1-(4-nitrophenyl)-5-methyl-5-(N-methyl-N-Tscarbamoylmethyl)-3-(2-propenylidene)cyclopentene (IC)
[0042]
[0043] Under nitrogen atmosphere, add 0.3mmol of 3-methyl-3-butene-1-ynamine compound, 0.36mmol of acetylenic alcohol, 3mL of 1,2-dichloroethane, PPh into a 10ml reaction tube 3 AuCl 0.03mmol, AgOTf 0.03mmol. React at 25°C for 4 hours to obtain the target product formula (IC), a pale yellow oily liquid with an isolated yield of 75%.
[0044] Product NMR data: 1H NMR (400MHz, CDCl 3 ): δ8.00(d, J=8.9Hz, 2H), 7.55(d, J=8.4Hz, 2H), 7.37(d, J=9.0Hz, 2H), 7.21(d, J=8.1Hz, 2H ),6.59(s,1H),3.10-3.14(m,4H),2.78(d,J=17.2Hz,1H),2.72(d,J=16.0Hz,1H),2.36-2.43(m,4H) ,1.77(s,3H),1.64(s,3H),1.32(s,3H); 13C NMR(100MHz,CDCl3):δ170.9,149.5,146.1,144.9,143.9,136.2,136.0,131.7,129.8,127.4, 126.9, 126.5, 123.3, 48.2, 44.6, 44.5, 32.8, 27.5, 21.4, 21.4, 20.7.
[0045] High resolution mass spectrometry data: HRMS(ESI,m / z)calcd.for C 25 h 29 N 2 o 5 S[M+H]+calc.:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com