Preparation method and application of covalent organic framework nanowire material
A covalent organic framework, nanowire technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of the reduction of the effective collision rate of the adsorption material and the reduction of the extraction capacity, achieve excellent optical activity, and avoid the limitation of adsorption capacity , the effect of strong photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
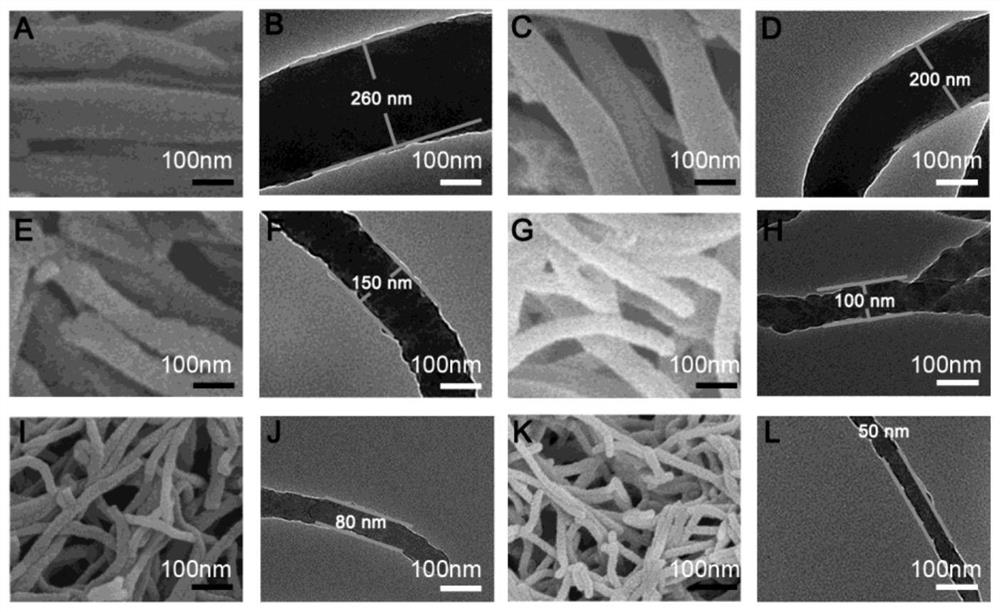
[0030] N 3 Preparation of -COF-Nws-1 covalent organic framework nanowire material
[0031] Inject 1,3,5-trialdehyde phenyltriazine (25mg, 0.065μmol) and 0.45mL dioxane, 0.45mL mesitylene, 0.1mL n-butanol and 0.1mL 6mol / L acetic acid solution into a 10mL pressure-resistant glass tube Obtain a suspension. Then hydrazine hydrate (5 μL, 50-60% solution) was added to the suspension, and then the pressure-resistant glass tube was sealed and heated at 120° C. for 72 hours. Take out the pressure-resistant glass tube, lower the temperature to room temperature, open the pressure-resistant glass tube, collect the precipitate formed, then filter and wash with anhydrous chloroform, anhydrous acetone and anhydrous tetrahydrofuran to obtain a powder sample, and put the powder sample at room temperature and Dry under vacuum to obtain light yellow powder covalent organic framework nanowire material N 3 -COF-Nws-1, its structural formula is as follows:
[0032]
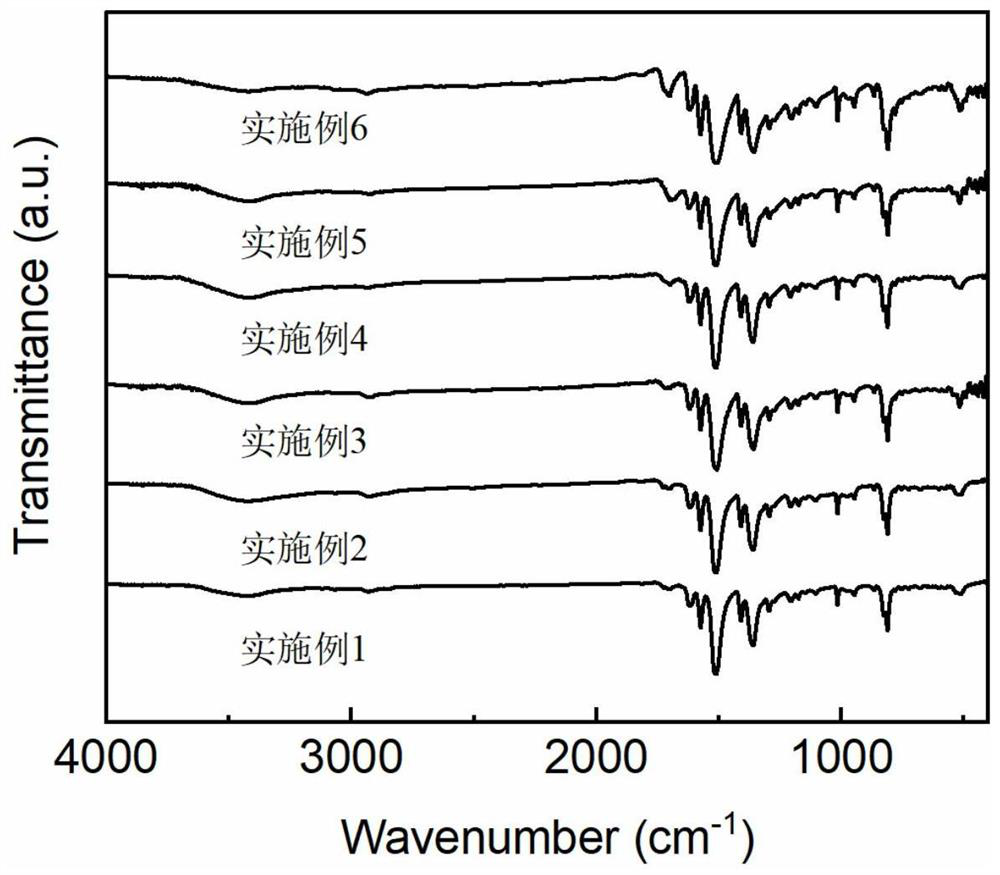
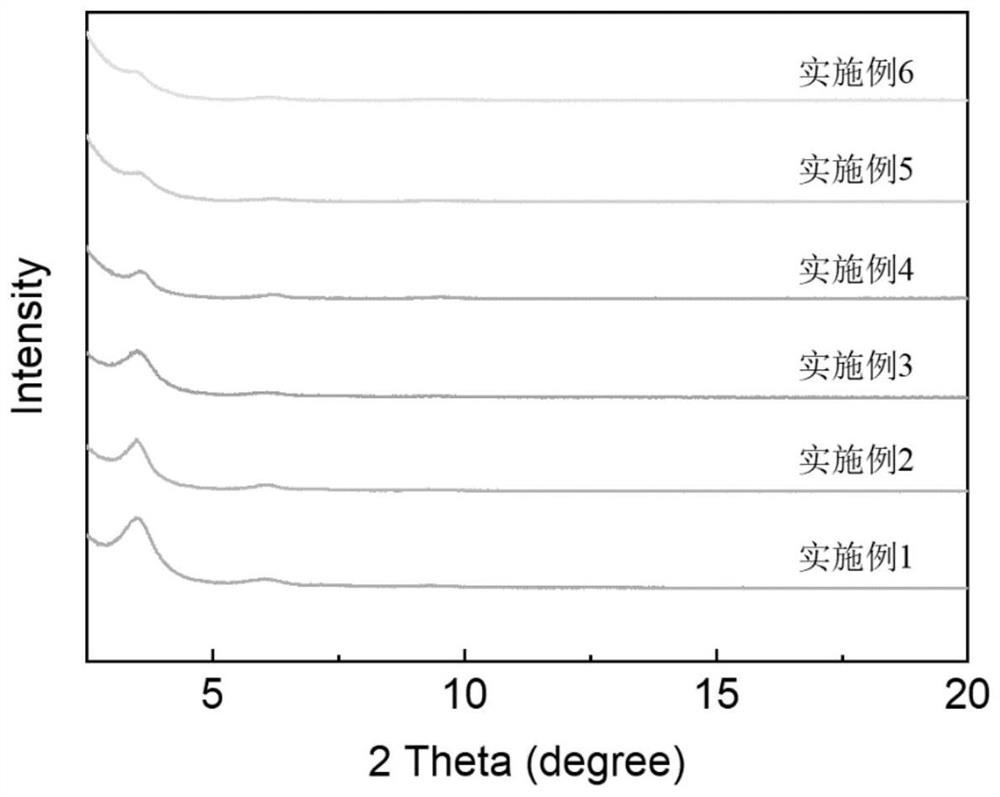
[0033] N 3 -Test steps ...
Embodiment 2
[0039] N 3 Preparation of -COF-Nws-2 covalent organic framework nanowire material
[0040] Inject 1,3,5-trialdehyde phenyltriazine (25mg, 0.065μmol) and 0.4mL dioxane, 0.4mL mesitylene, 0.2mL n-butanol and 0.1mL 6mol / L acetic acid into a 10mL pressure-resistant glass tube The solution obtained a suspension. Then hydrazine hydrate (5 μL, 50-60% solution) was added to the suspension, and then the pressure-resistant glass tube was sealed and heated at 120° C. for 72 hours. Take out the pressure-resistant glass tube, lower the temperature to room temperature, open the pressure-resistant glass tube, collect the formed precipitate, filter it with anhydrous chloroform, anhydrous acetone and anhydrous tetrahydrofuran, and obtain a powder sample after washing. The powder sample was dried under vacuum at room temperature to obtain a light yellow powder covalent organic framework nanowire material N 3 -COF-Nws-2, its structural formula is as follows:
[0041]
[0042] N 3 -Test s...
Embodiment 3
[0044] N 3 - Preparation of COF-Nws-3 covalent organic framework nanowire material
[0045] Inject 1,3,5-trialdehyde phenyltriazine (25mg, 0.065μmol) and 0.3mL dioxane, 0.3mL mesitylene, 0.4mL n-butanol and 0.1mL 6mol / L acetic acid into a 10mL pressure-resistant glass tube The solution obtained a suspension. Then hydrazine hydrate (5 μL, 50-60% solution) was added to the suspension, and then the pressure-resistant glass tube was sealed and heated at 120° C. for 72 hours. Take out the pressure-resistant glass tube, lower the temperature to room temperature, open the pressure-resistant glass tube, collect the formed precipitate, then filter and wash with anhydrous chloroform, anhydrous acetone and anhydrous tetrahydrofuran to obtain a powder sample. The powder sample was dried under vacuum at room temperature to obtain a light yellow powder covalent organic framework nanowire material N 3 -COF-Nws-3, its structural formula is as follows:
[0046]
[0047] N 3 -Test steps...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com