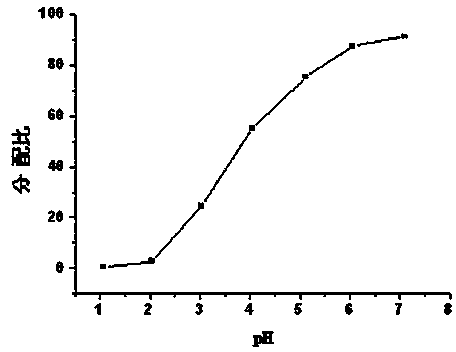
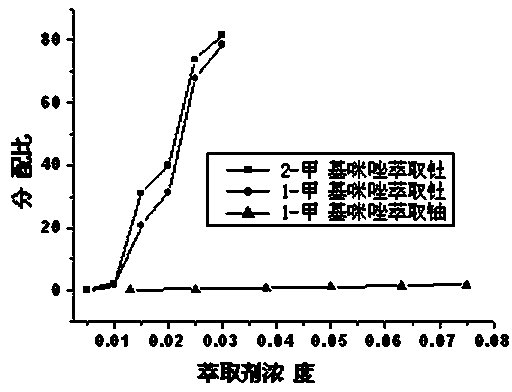
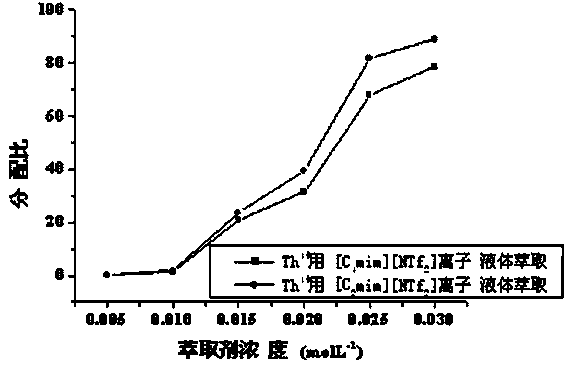
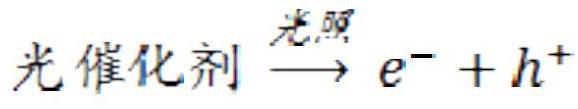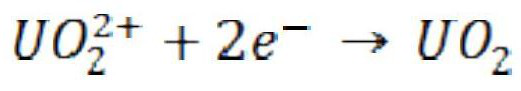Patents
Literature
223 results about "Uranous" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uranous is the chemical term for the reduced tetrapositive cation of uranium that exhibits the valence U⁴⁺. It is one of the two common ionic states of uranium found in nature, the other being the oxidised hexapositive ion called uranyl. Uranous compounds are usually unstable; they revert to the oxidised form on exposure to air.
Leaching of mineral ores
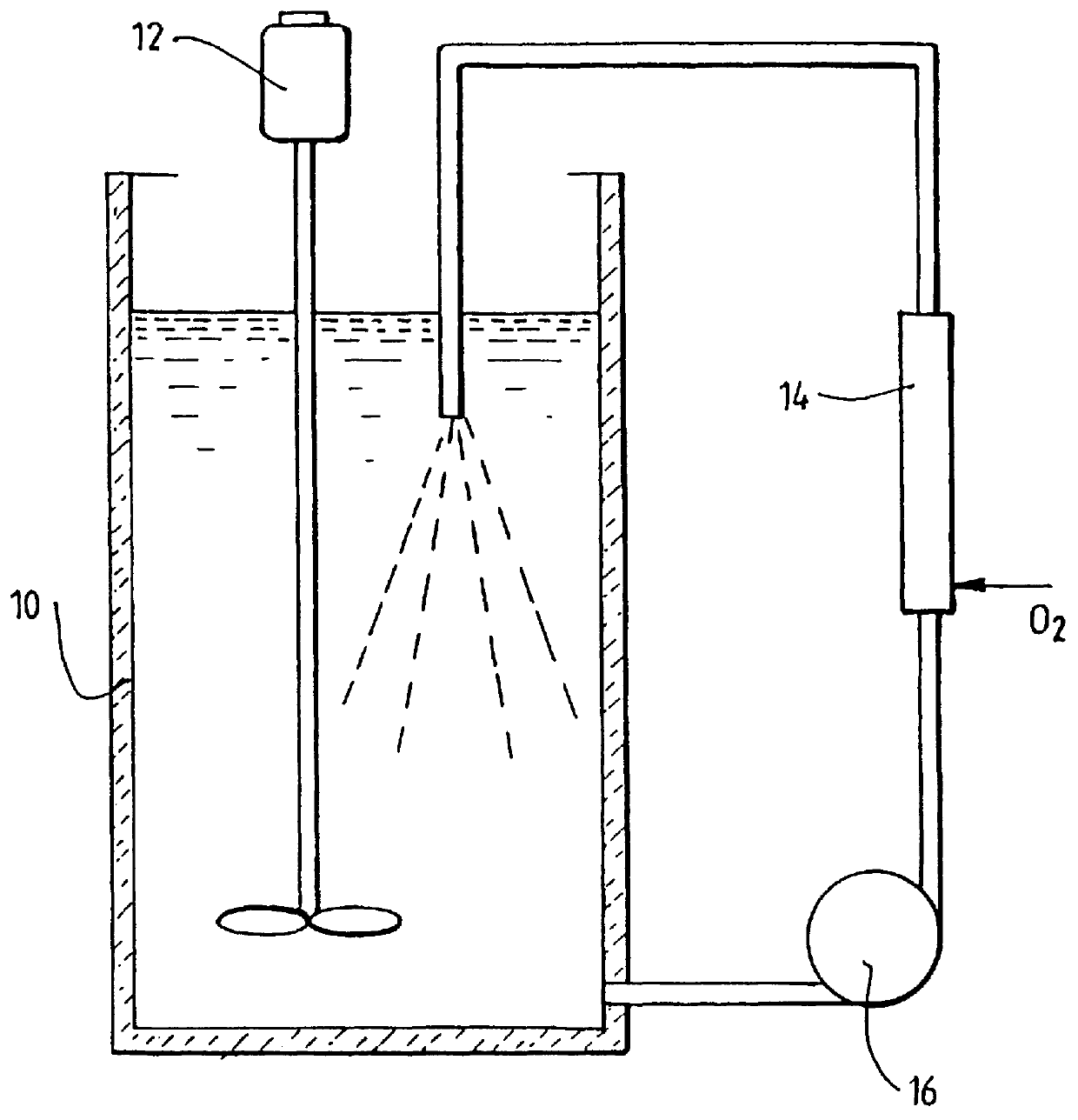
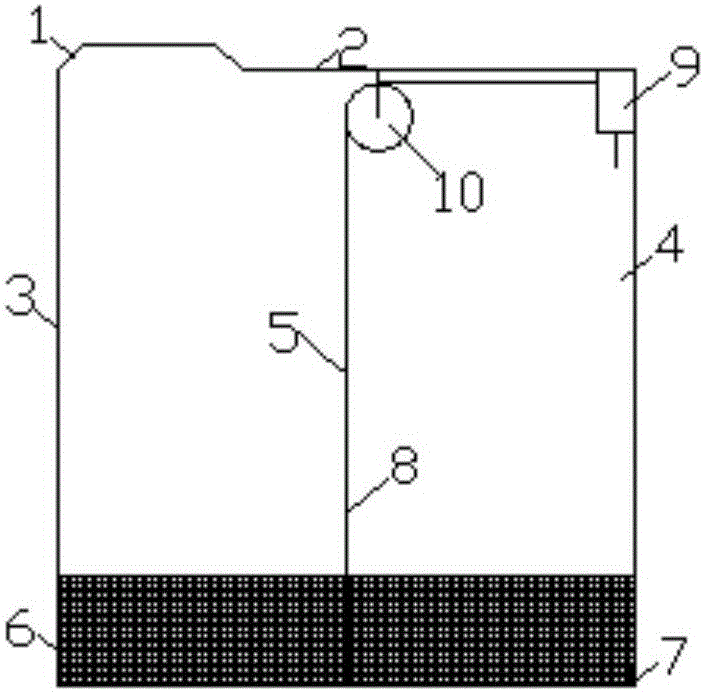
A process for oxidation of ferrous ions in solution, and more particularly a process for improved base metal and / or uranium leaching from ores, concentrates or tailings using ferric ion as an oxidizing agent. A reaction vessel (10) holds a ferrous ion-containing solution, for example, a copper sulphide leach slurry or concentrate. An agitator (12) may be provided to promote leaching of the base metal into solution. Some of the ferrous ion-containing solution is drawn off from the reaction vessel (10) and pumped through an in-line mixer (14) via a feed pump (16). Oxygen is injected into the reactor (14) to facilitate oxidation of the ferrous sulphate to form ferric sulphate. The ferric ion-containing solution is then recirculated back to the reaction vessel (10) where the ferric ions are reused in the dissolution of copper sulphide into soluble copper sulphate. The two processes of ferrous oxidation and metal leaching can be conducted either simultaneously or sequentially to effect recovery of copper, other base metals or uranium from ores, concentrates or tailings.
Owner:ATOMAER
Method for extracting uranium from uranium-contained niobium-tantalum leached tailings
ActiveCN102312094AReduce consumptionReduce energy consumptionProcess efficiency improvementNiobiumEnergy consumption
The invention relates to a method for extracting uranium from uranium-contained niobium-tantalum leached tailings, which comprises the steps of sulfuric acid leaching, extraction, back extraction, purification, extraction and purification, back extraction and the like. Compared with the prior art, the method for extracting the uranium from the uranium-contained niobium-tantalum leached tailings is capable of reducing the raw material consumption; pretreatment processes of exploitation, mineral separation and the like are not needed; and in a practical process, energy consumption is reduced, the process problem of extracting the uranium from complicated substrates is solved, and a qualified uranium product is successfully manufactured.
Owner:BEIJING RES INST OF URANIUM GEOLOGY
Solvent extraction process for recovery of uranium from phosphoric acid (25-55% P2O5)
InactiveUS20030113247A1Cheaply and stablySpeed up the processSolvent extractionTransuranic element compoundsPhosphorous acidO-Phosphoric Acid
An improved process of extraction of uranium from phosphoric acid and in particular uranium VI from phosphoric acid especially strong phosphoric acid using a selective synergistic extractant mix of an organo-phosphorous acid and a neutral extraction agent. The process basically involves the steps of extraction comprising contacting said acid with a selective synergistic extractant system of di-nonyl phenyl phosphoric acid (DNPPA) and a neutral agent selected from di-butyl butyl phosphonate (DBBP) and tri-n-octyl phosphine oxide (TOPO); and recovering the uranium values from the loaded organic phase. The above process would provide for an improved process for recovery of uranium both from weak and strong phosphoric acids using a stable and relatively cheap extractant system. The process is directed to improved recovery of U-VI from phosphoric acid by way of a simple, industrially applicable and cost-effective process.
Owner:SEC DEPT OF ATOMIC ENERGY
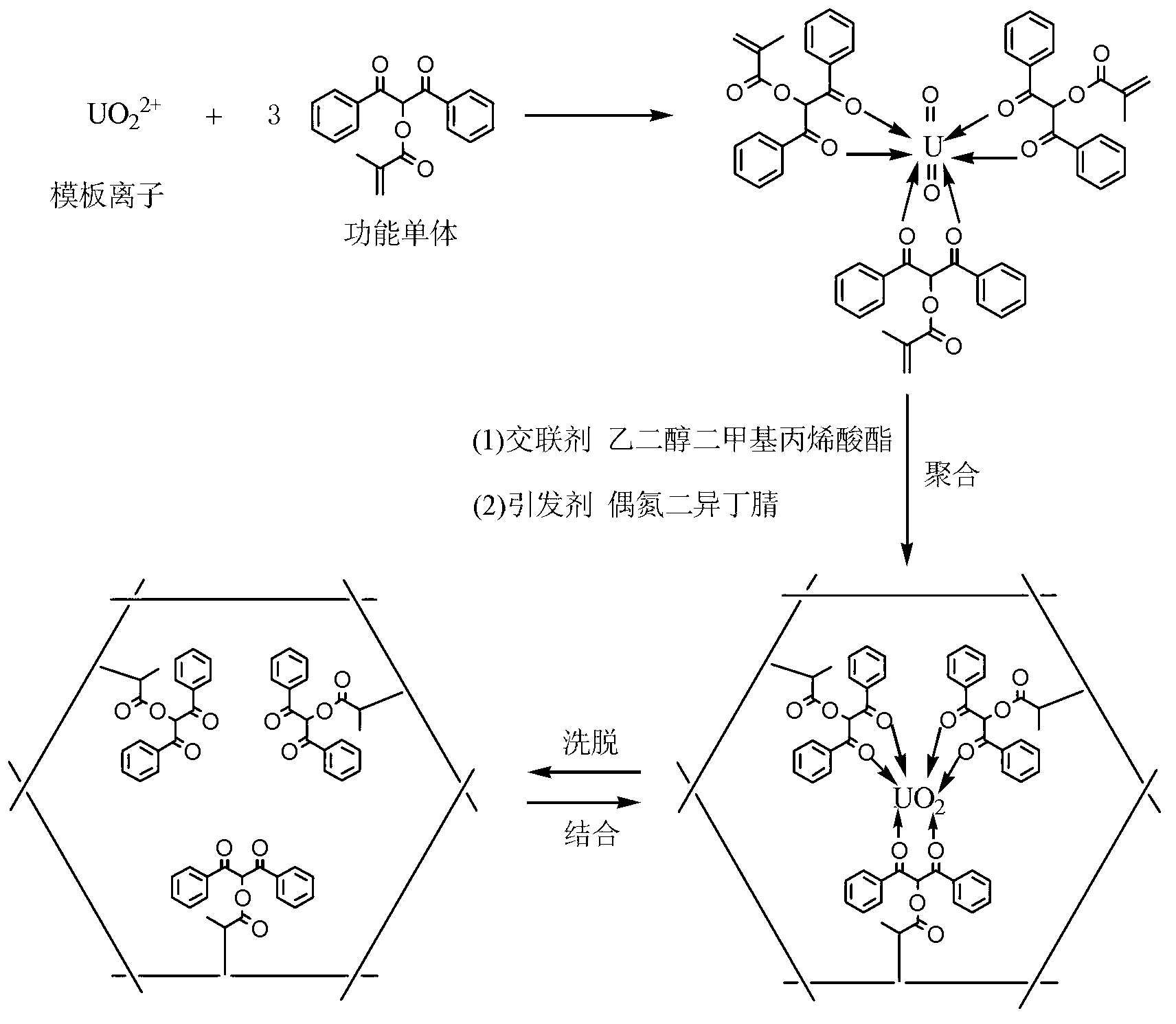
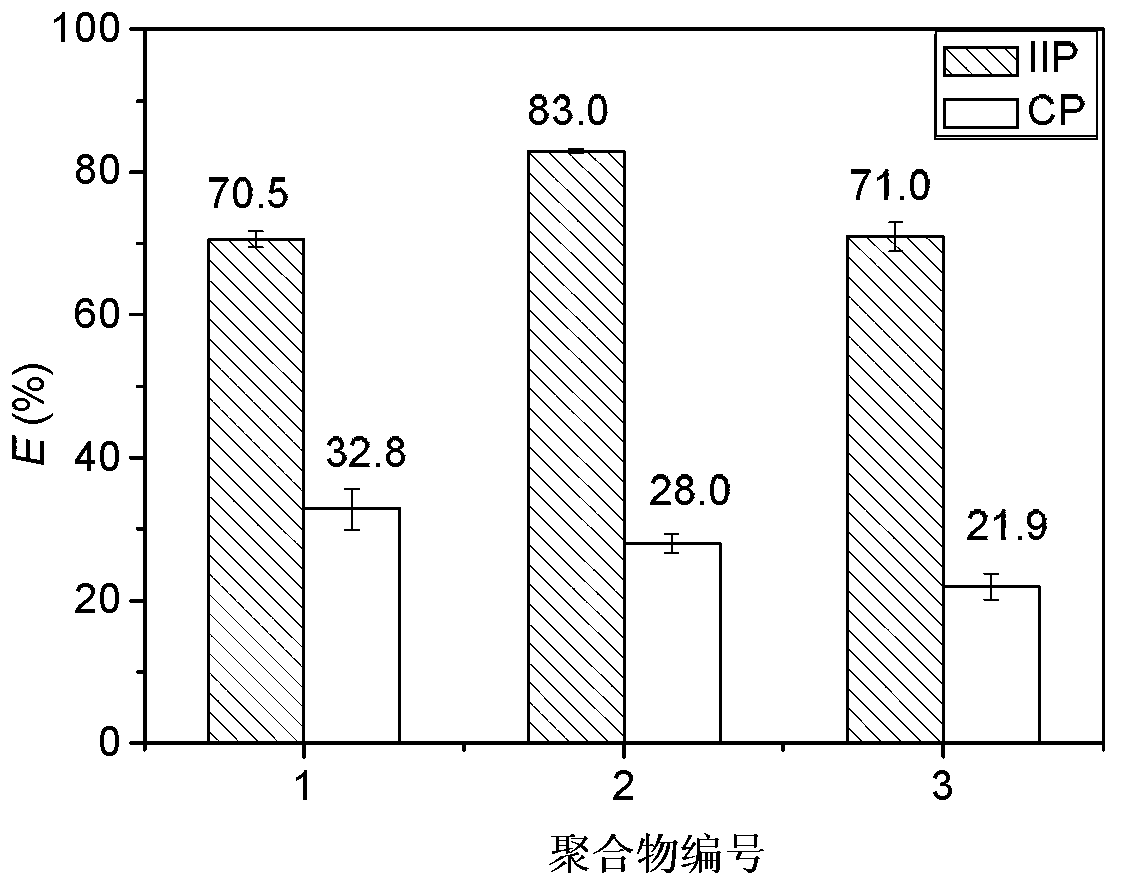
Uranyl ion imprinted polymer and preparation method and application thereof
The invention discloses a uranyl ion imprinted polymer and a preparation method and application thereof. The uranyl ion imprinted polymer is prepared by the steps of with a uranyl ion as a template and beta-diketone modified with acryloyl group or methacryloyl as a functional monomer, carrying out polymerization reaction under the actions of an initiator and a cross-linking agent, and removing the template uranyl ion from the obtained polymer after the reaction is ended. The imprinted polymer can compete to absorb uranyl ions from uranyl ammonium carbonate containing a lot of free carbonates, is high in adsorption capacity, and is not interfered by metal ions such as Li<+>, Na<+>, K<+> and Rb<+>. Therefore, the imprinted polymer can be used for adsorbing and extracting uranium form sea water with complicated ingredients and low uranium concentration.
Owner:PEKING UNIV
Preparation method of thorium-uranium mixed oxide ceramic microspheres
ActiveCN103165206ANo stringent requirementsFlat surfaceNuclear energy generationReactors manufactureOxide ceramicMixed oxide
The invention belongs to a preparation method of nuclear fuel, and concretely relates to a preparation method of thorium-uranium mixed oxide ceramic microspheres. The method uses an internal gelation method, and comprises using an acid-lacking uranyl nitrate solution and thorium nitrate as raw materials, preparing sol, dispersing gelling, washing, drying, calcining and reduction sintering to prepare ThO2-UO2 microspheres with a diameter of 0.05-0.20 mm. The ThO2-UO2 microspheres has outstanding advantages of smooth surface, good sphericity, compact inside, uniformly distributed thorium, and a density being greater than 96.8% TD; and the method is simple, and can prepare the thorium-uranium mixed oxide ceramic microspheres with different thorium contents.
Owner:NUCLEAR POWER INSTITUTE OF CHINA
Ion sieve for extracting uranium from water body and preparation method thereof
InactiveCN103894155AOther chemical processesRadioactive decontaminationPhosphomolybdic acidHydroxylamine
The invention provides an ion sieve for extracting uranium from a water body and a preparation method thereof. The ion sieve is prepared from pyrophosphate, molybdate, zirconium oxychloride, hexadecyl trimethyl ammonium bromide, acrylonitrile, and hydroxylamine hydrochloride. The preparation method comprises the steps: preparing a hydrogen ion exchanger of zirconyl-molybdopyrophosphate polyoxometalate by using zirconium oxychloride, the molybdate and the pyrophosphate, introducing a defined amount of uranium ions to the hydrogen ion exchanger, extracting through immobilizing the uranium ions, and baking for forming; then radiating and activating, making a product obtained by radiating and activating react with hexadecyl trimethyl ammonium bromide for performing organic modification, and then adding acrylonitrile and hydroxylamine hydrochloride for performing amine oximation; finally, performing solid-liquid separation, and then performing steps of high-temperature sintering, cooling and grinding, and the like to obtain the ion sieve for extracting uranium from the water body. The prepared ion sieve has a most suitable crystal structure of receiving the uranium ions, shows an efficient selective effect, and has a chelation function and a good selectivity to the uranium ions.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
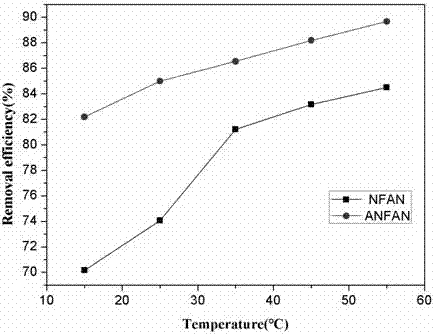
Method for efficiently removing and recovering uranium in water by utilizing titanium-based titanium dioxide nanotube array electrode
ActiveCN109867333AEfficient and fast removalEfficient and fast recyclingWater/sewage treatment using germicide/oligodynamic-processRadioactive decontaminationHigh concentrationOxygen
The invention discloses a method for efficiently removing and recovering uranium in water by utilizing a titanium-based titanium dioxide nanotube array electrode. The method adopts an anatase phase titanium dioxide nanotube array electrode and a stable good-conductivity material as a cathode and an anode respectively to construct an electrochemical reduction system, hexavalent uranium in a solution is subjected to electrochemical reduction to form tetravalent uranium by utilizing a stronger coordination effect of anatase phase titanium dioxide and uranyl ions in the absence of external additives, and the tetravalent uranium adheres to the electrode surfaces; and after electrochemical reduction enrichment is completed, a tetravalent uranium-rich anatase phase titanium dioxide nanotube arrayelectrode is taken out from the solution, so that high-efficiency reduction removal of the uranium in the wastewater, the groundwater and / or the seawater can be realized. The method provided by the invention has a wide application range, and can realize high-efficiency removal and recover of the uranium for the wastewater, the groundwater and the seawater containing a high concentration of dissolved oxygen, a high concentration of a carbonate and a low concentration of the uranium.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
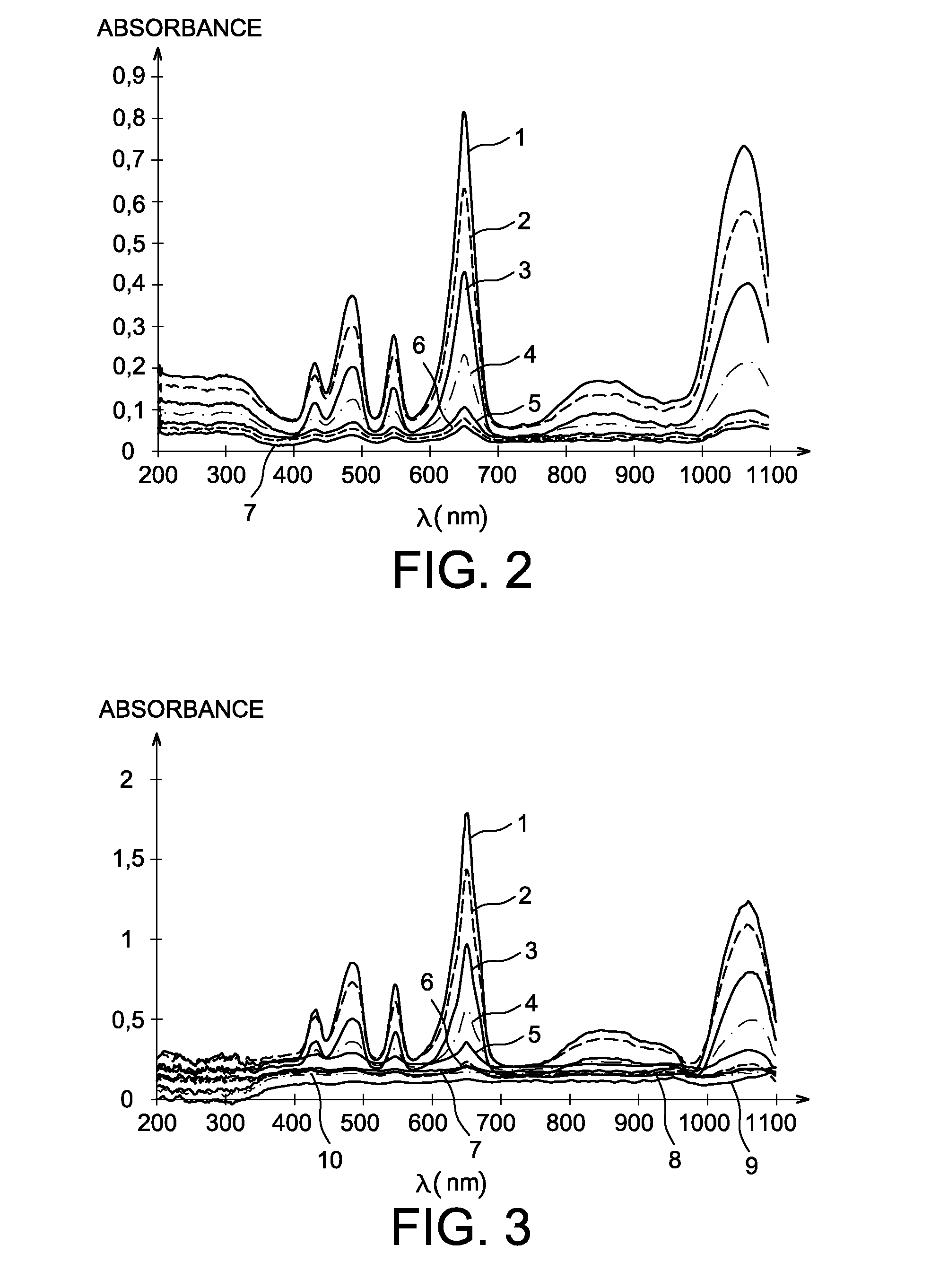
Method for measuring the uranium concentration of an aqueous solution by spectrophotometry
InactiveUS20130206599A1Less interferenceRadiation pyrometryPhotography auxillary processesSpectrophotometryAqueous solution
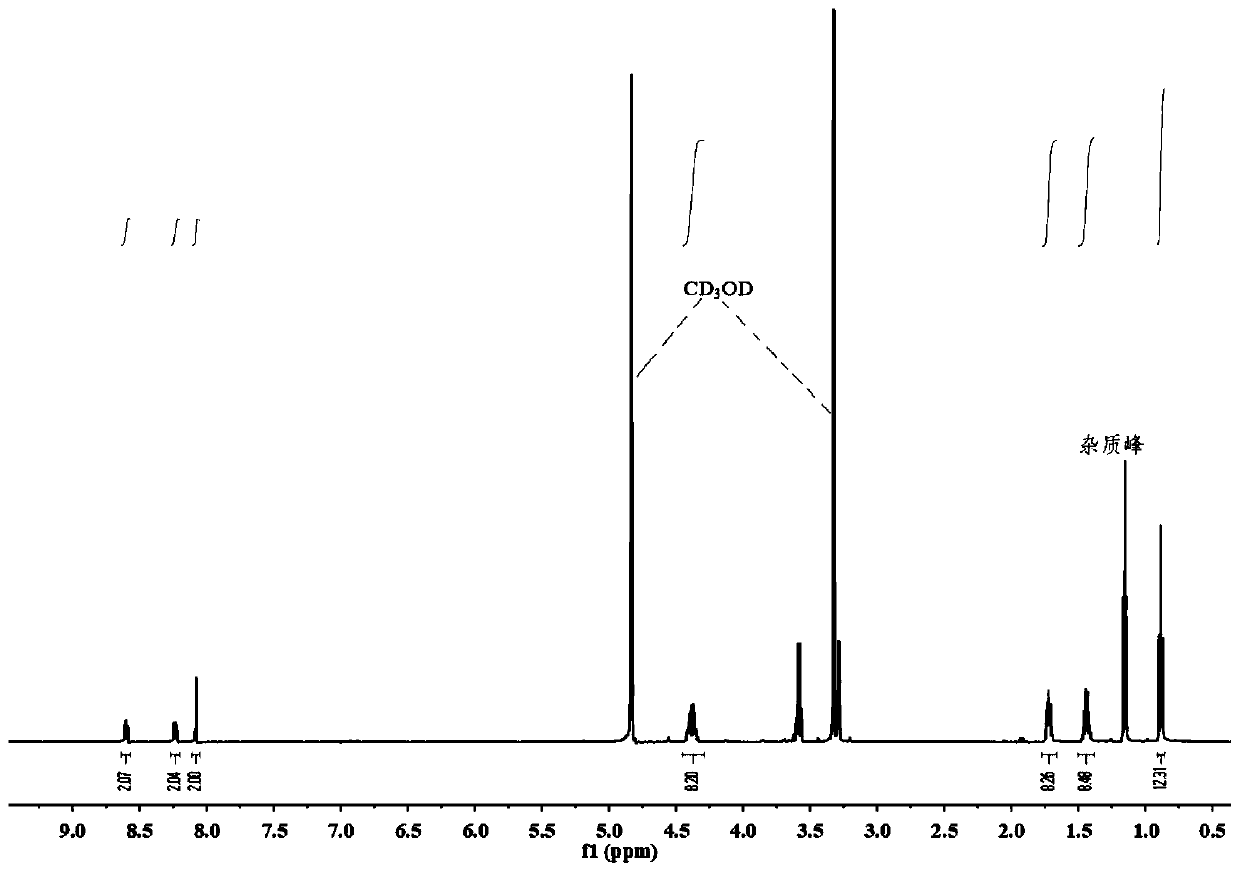
A method for measuring the uranium concentration of an aqueous solution including the following successive steps: a) electrochemical reduction towards valence IV, of the uranium present in the aqueous solution with a valence greater than IV, this reduction being implemented at pH<2 and by passing an electrical current in the solution; b) measurement of the absorbance of the solution obtained on completion of step a) at a chosen wavelength between 640 and 660 nm, and preferably 652 nm; and c) determination of the uranium concentration of the aqueous solution by deduction of the uranium concentration of valence (IV) present in the aqueous solution obtained on completion of step a) from measurement of the absorbance obtained in step b).
Owner:AREVA NC
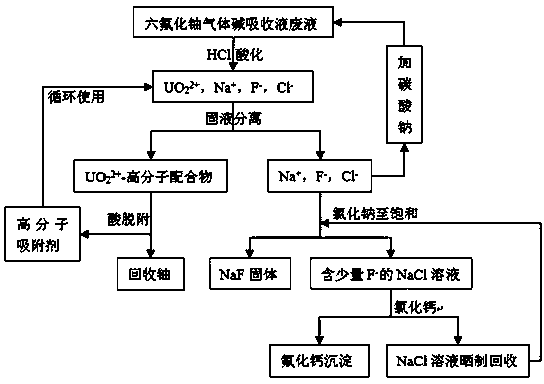
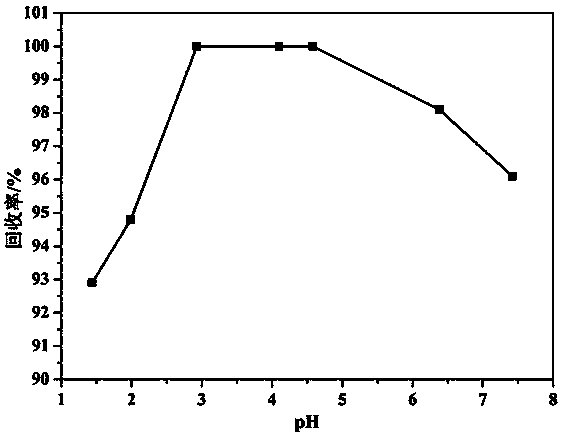
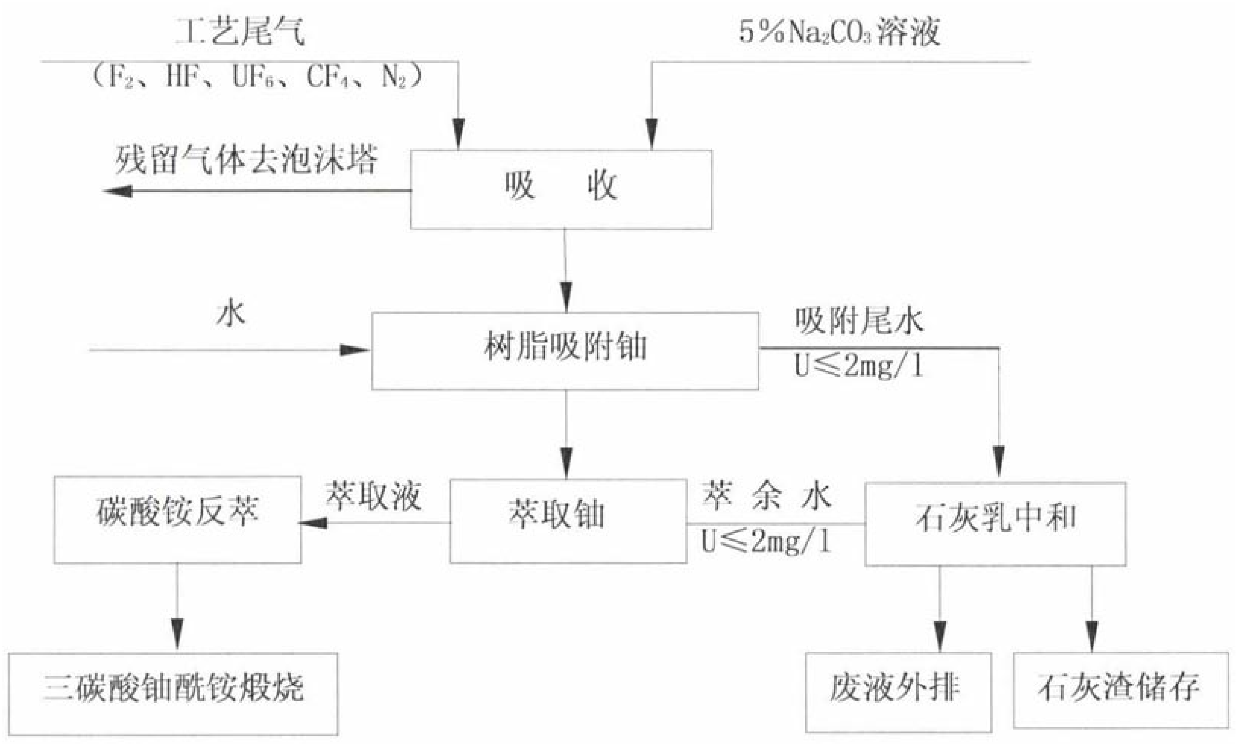
Recovering method for uranium and fluorine in uranium hexafluoride alkali absorption solution waste liquid
ActiveCN103886925AAchieve recyclingAchieving zero emissionsCalcium/strontium/barium fluoridesAlkali metal fluoridesSorbentDesorption
The invention discloses a recovering method for uranium and fluorine in uranium hexafluoride alkali absorption solution waste liquid. According to the recovering method, the uranium hexafluoride alkali absorption solution waste liquid is firstly acidized through acid, the pH is equal to 2.7-5.5 through adjustment, then macromolecule adsorbent (polystyrene-cyclohexyl amino maleic acid) is added to finish UO22+ adsorption, solid-liquid separation is performed, so that desorption is performed on obtained solids through acid with concentration larger than 1M to recover UO22+, Na2CO3 is added into the remaining solution to perform a next cycle of uranium hexafluoride elution, and after five or more cycles, the solution is rich in F- high in concentration. NaC1 is added into the solution, so that the solution is saturated, and NaF is precipitated due to the fact that the solubility is reduced, NaF solids are obtained through filtering and recovering, CaC12, or CaO or Ca (OH)2 solids or saturated solutions are then added into the solution, so that the residual F- forms CaF2 sediment, and fluoride removal is performed deeply. Finally, the uranium and the large amount of fluoride in the uranium hexafluoride alkali absorption solution waste liquid are recovered. Uranium in the radioactive waste liquid is recovered, zero drainage of the waste liquid is achieved, the drainage of the waste liquid reaches the national standard, and meanwhile the problem that in a traditional fluoride removal technology, a large amount of calcium fluoride is difficult to filter is solved.
Owner:南京京科新材料研究院有限公司
Preparation method of cellulose-based amidoximation seawater uranium extraction adsorption microspheres
PendingCN112892490AWide variety of sourcesLow costOther chemical processesAlkali metal oxides/hydroxidesHydroxylamineMicrosphere
The invention discloses a preparation method of cellulose-based amidoximation seawater uranium extraction adsorption microspheres. The method comprises the following steps: dissolving a cellulose-based macromolecular substance in an alkali and urea system solution, and violently stirring under a low-temperature condition until the cellulose-based macromolecular substance is completely dissolved and forms a homogeneous solution; then dropwise adding acrylonitrile into the homogeneous solution, reacting in an ice-water bath environment, centrifugally collecting the lower insoluble substance after the reaction is finished, washing the insoluble substance with pure water for multiple times to remove impurities, and drying to obtain cyanoethyl cellulose; and dissolving the obtained cyanoethyl cellulose in an organic solvent, then adding hydroxylamine hydrochloride and inorganic alkali into the solution to react, and dropwise adding the solution into a coagulating bath solution to obtain a product result. The preparation method is easy to operate, high in controllability and easy for industrial large-scale production, and the prepared product can be applied to the aspects of uranium extraction from seawater, heavy metal ion adsorption and gold reduction and enrichment.
Owner:ZHEJIANG SCI-TECH UNIV
Preparation of amidoxime modified magnetic nano biological adsorbent and method for adsorbing low-concentration uranium by utilizing amidoxime modified magnetic nano biological adsorbent
ActiveCN107537455AHigh adsorption rateThe adsorption rate is stableOther chemical processesAlkali metal oxides/hydroxidesCross-linkSorbent
The invention relates to preparation of an amidoxime modified magnetic nano biological adsorbent and a method for adsorbing low-concentration uranium by utilizing the amidoxime modified magnetic nanobiological adsorbent. The preparation comprises the following steps: modifying magnetic nano Fe3O4 by utilizing amidoxime; grafting the amidoxime modified nano Fe3O4 on aspergillus niger through cross-linking reaction to obtain an amidoxime modified magnetic nano Fe3O4-aspergillus niger biological adsorbent. Lone pair electrons on an electron-donating group of the amidoxime based adsorbent and uranyl ions can form a coordination bond or a stable structure, so that the adsorption speed and the adsorption capacity of the material on the uranyl ions can be effectively improved. The uranium compatible property of the aspergillus niger, the magnetic property of the nano Fe3O4 and the high selectivity and affinity of an amidoxime group on the uranium are combined and the aspergillus niger is a biological material, so that the amidoxime modified magnetic nano biological adsorbent has the advantages of good adsorption property, simplicity in operation, low production cost, low energy consumption, easiness for solid-liquid separation and the like; the product has a good application prospect in the fields of prevention and control of radioactive pollution and uranium cyclic utilization.
Owner:NANHUA UNIV
Membrane treatment process for purifying uranium-containing waste liquid
InactiveCN107456873AReduce energy consumptionLess secondary wasteSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisReverse osmosisUranium hexafluoride
The invention belongs to the field of uranium conversion, and particularly relates to a membrane purification treatment process for uranium-containing process waste liquid generated in a production process of uranic fluoride. The membrane purification treatment process comprises the following steps: (1) establishing a uranium-containing waste liquid treatment purification system; (2) carrying out pretreatment; (3) carrying out ultrafiltration; (4) carrying out nanofiltration; and (5) carrying out reverse osmosis. By establishment of the membrane purification treatment system for the uranium-containing waste liquid, after the uranium-containing waste liquid of which the uranium concentration is about 200-800 mg / L in a uranium purification and conversion system is separated by a nanofiltration unit (under the operation pressure of 0.3 MPa) and a reverse osmosis unit (under the operation pressure of 1.0 MPa), the concentration of uranium in permeate can be reduced to be 0.05 mg / L or below, and therefore the permeate reaches the emission standard. In addition, the membrane treatment technology has the advantages of low energy consumption, simple system, less secondary wastes and the like.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
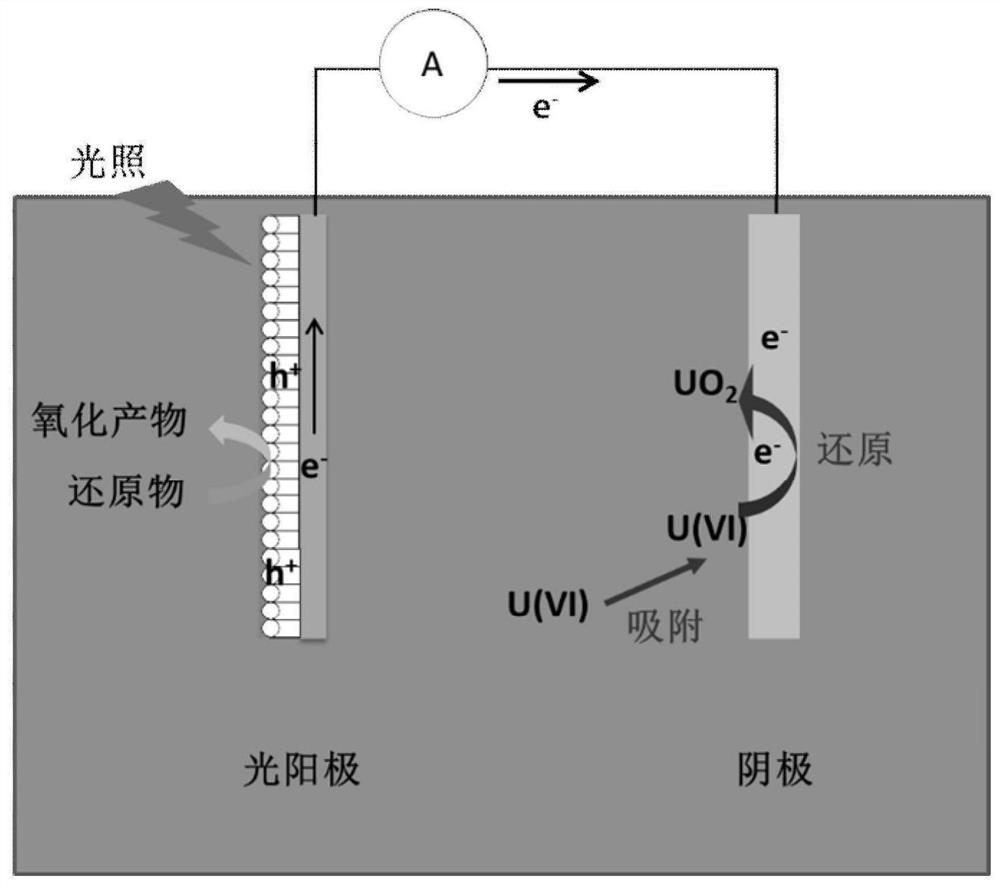
Device and method for extracting uranium from uranium-containing wastewater or seawater and application thereof
ActiveCN112342385AExtended regeneration cycleReduce extraction costsProcess efficiency improvementPhotoelectrochemical storage cellsAir atmosphereCathodic reaction
The invention discloses a device, method and application for extracting uranium from uranium-containing wastewater or seawater, and the device for extracting the uranium comprises a photo-anode, a cathode reaction piece and a photocatalytic light source. The photo-anode comprises an anode reaction piece and a photocatalyst layer arranged on the surface of the anode reaction piece. The cathode reaction piece is connected with the anode reaction piece through a wire. The photocatalytic light source adopts ultraviolet light, visible light or infrared light. When a photocatalytic fuel cell is usedfor extracting the uranium from the uranium-containing wastewater and seawater, an external power source is not needed, a proton exchange membrane and an oxide inhibitor do not need to be added, meanwhile, inert gas atmosphere protection is not needed, and operation can be conducted in the air atmosphere. Hexavalent uranyl ions in the wastewater and seawater can be efficiently reduced into tetravalent uranium which is low in toxicity and almost insoluble in water, the tetravalent uranium is gathered onto a cathode, uranium is desorbed from the surface of a photocatalyst without acid picklingor air purging reoxidation, uranium can be efficiently recycled by regularly replacing a cathode material, uranium reduction products are easy and convenient to collect, and continuous operation of asystem is not influenced.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
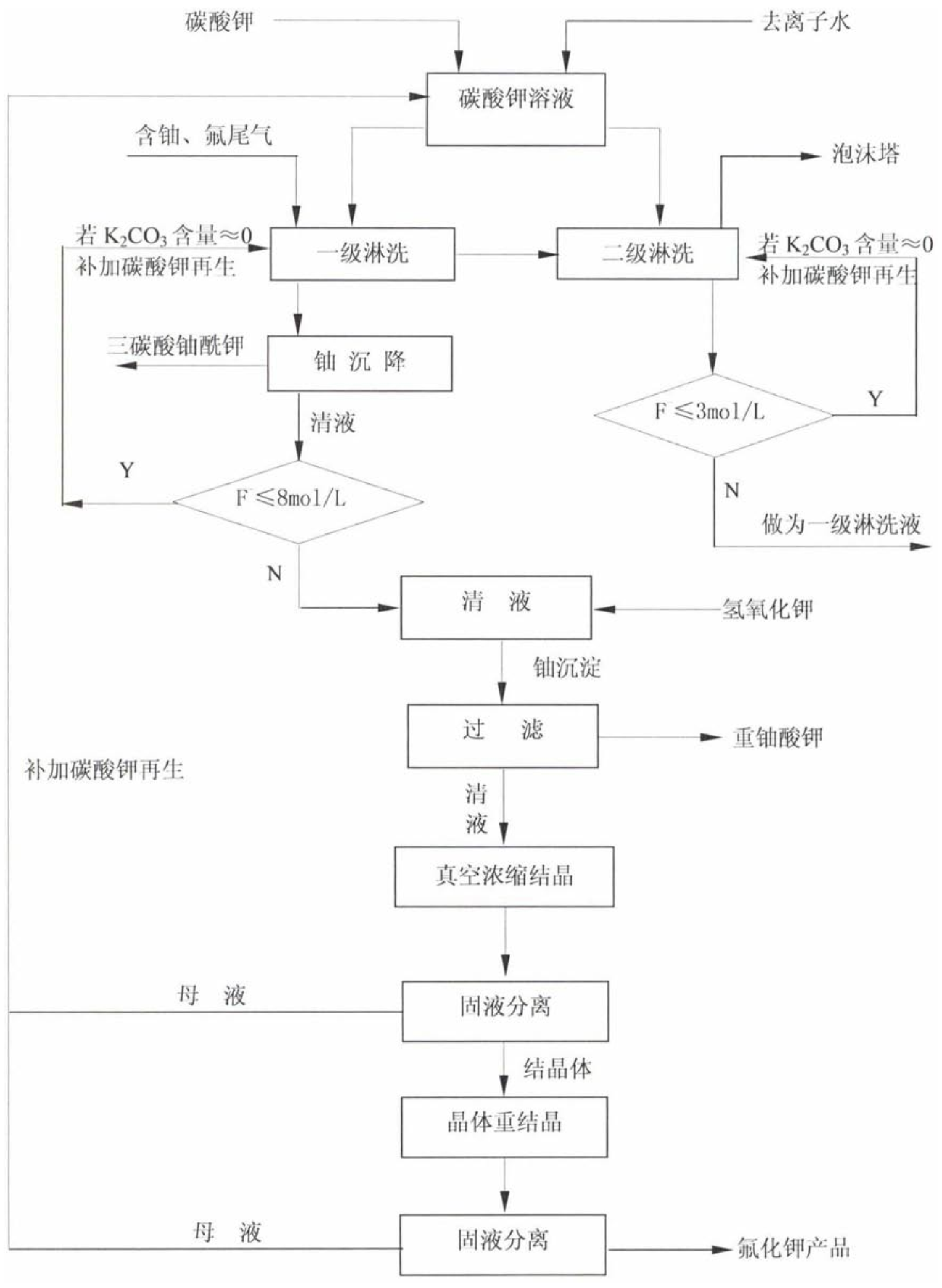
Uranium and fluorine-containing tail gas leaching and eluent regeneration process
ActiveCN106508071BSave resourcesTo achieve the goal of "turning waste into treasure"Uranium fluoridesDispersed particle separationChemical industryFiltration
The invention relates to a process for treating uranium and fluorine-containing tail gas and waste liquid in the field of uranium chemical industry, in particular to a uranium- and fluorine-containing tail gas leaching and eluent regeneration process, which is carried out in sequence as follows: (1) K2CO3 solution is used for leaching Tail gas containing uranium and fluorine; (2) Potassium uranyl tricarbonate is separated by sedimentation. If the mass percentage concentration of K2CO3 in the resulting clear liquid is ≈0 and the concentration of KF is >8mol / l, add KOH to the clear liquid to generate diuranic acid Potassium precipitation; (3) Potassium diuranate precipitate is separated by filtration, and the obtained clear liquid is crystallized, and the crystal is KF crystal. The process of the invention can reduce the impact of radioactive waste on the environment, and has good social benefits; the closed loop of the eluent avoids the loss of uranium elements, saves resources, and recycles fluorine ions in the form of KF by-products to achieve It achieves the purpose of "turning waste into treasure" and has certain economic benefits.
Owner:CNNC LANZHOU URANIUM ENRICHMENT
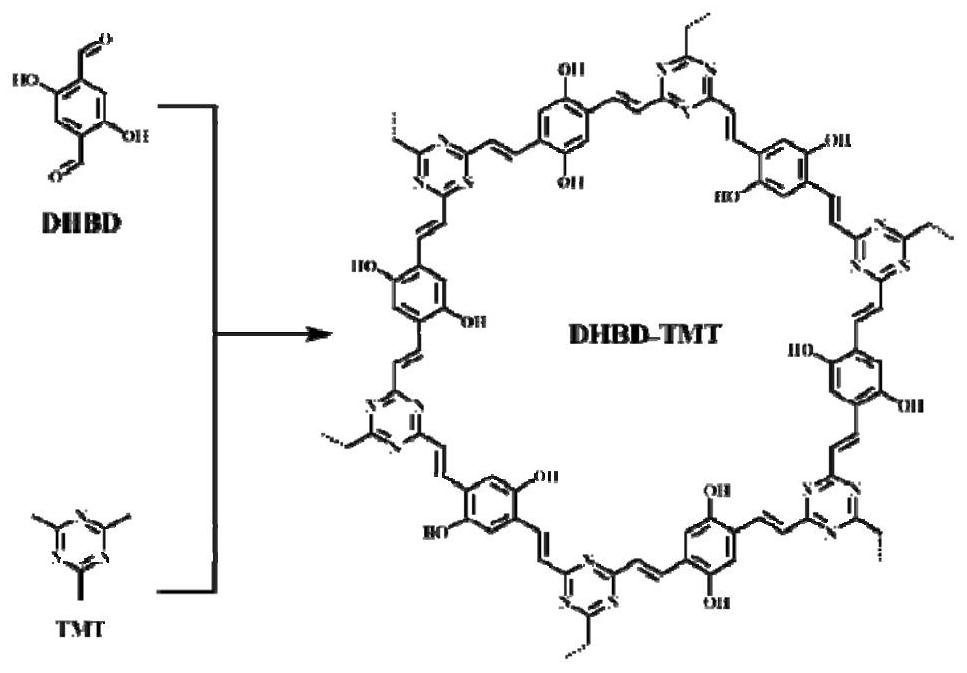
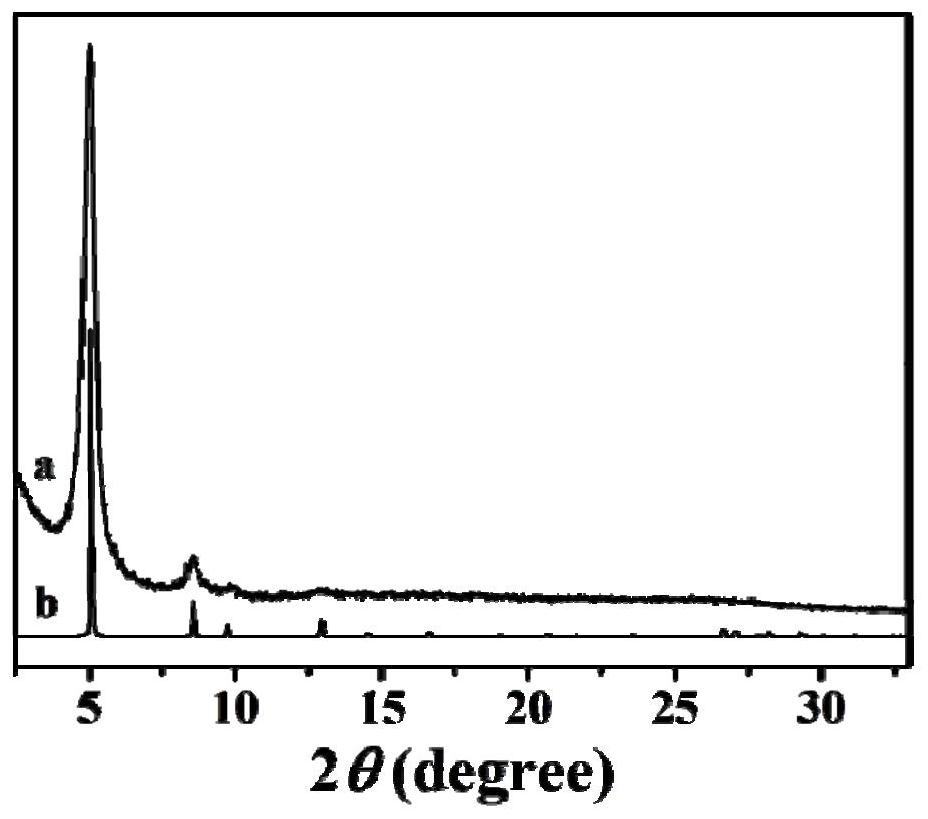
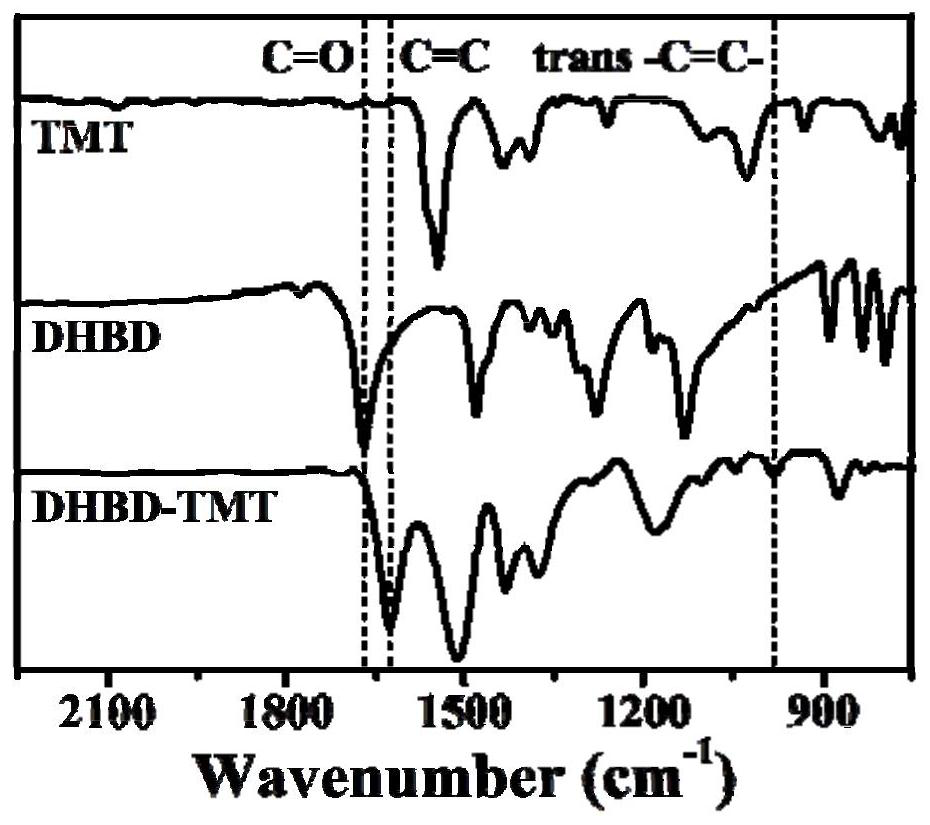
Preparation method of porous covalent organic framework and application of porous covalent organic framework in uranyl ion capture
ActiveCN113045723AAchieve fixationHigh adsorption capacityIon-exchange process apparatusOther chemical processesInductively coupled plasmaMethyl group
The invention discloses a preparation method of a porous covalent organic framework and application of the porous covalent organic framework in uranyl ion capture, belonging to the technical field of adsorption materials. The preparation method comprises the following step: carrying out an aldol condensation reaction on 2,5-dihydroxy-1,4-phthalaldehyde (DHBD) and 2,4,6-trimethyl-1,3,5-triazine (TMT) to prepare an olefinic bond porous covalent organic framework (DHBD-TMT). The DHBD-TMT prepared by the method disclosed by the invention has a large number of hydroxyl functional groups and a highly planar pi-conjugated structure, and can simultaneously realize multiple functions of selective adsorption, chemical reduction, photocatalytic reduction of uranium and the like, so the adsorption capacity of uranium is remarkably improved. Inductively coupled plasma mass spectrometry results show that DHBD-TMT has excellent adsorption performance on uranyl ions, and has the advantages of high adsorption capacity, good selectivity, excellent optical activity, high stability, good hydrophilicity and the like. Besides, the DHBD-TMT can reduce soluble uranium (VI) into insoluble uranium (IV) through chemical reduction and photocatalytic reduction under visible light irradiation, so uranium fixation is realized; and the DHBD-TMT is an efficient adsorbent for extracting uranyl ions.
Owner:NANCHANG UNIV
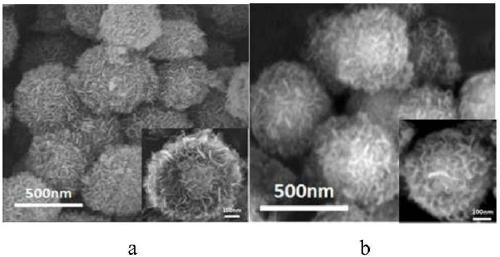
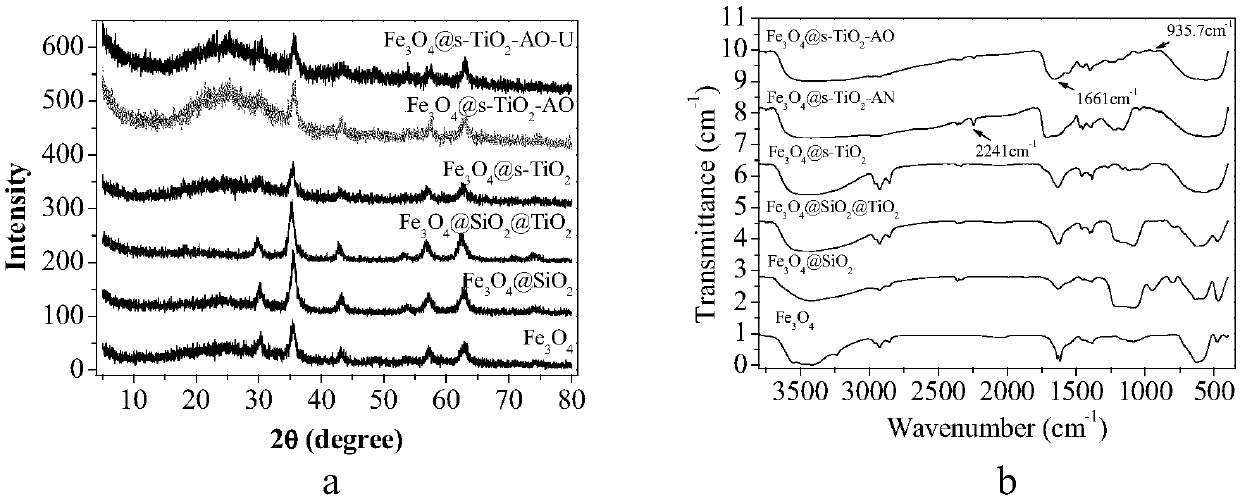
Magnetic nano functional material for extracting uranium from seawater, and preparation method thereof
ActiveCN109569548ANot easy to reuniteStrong magnetismOther chemical processesSeawater treatmentCitrinin hydrateHydroxylamine Hydrochloride
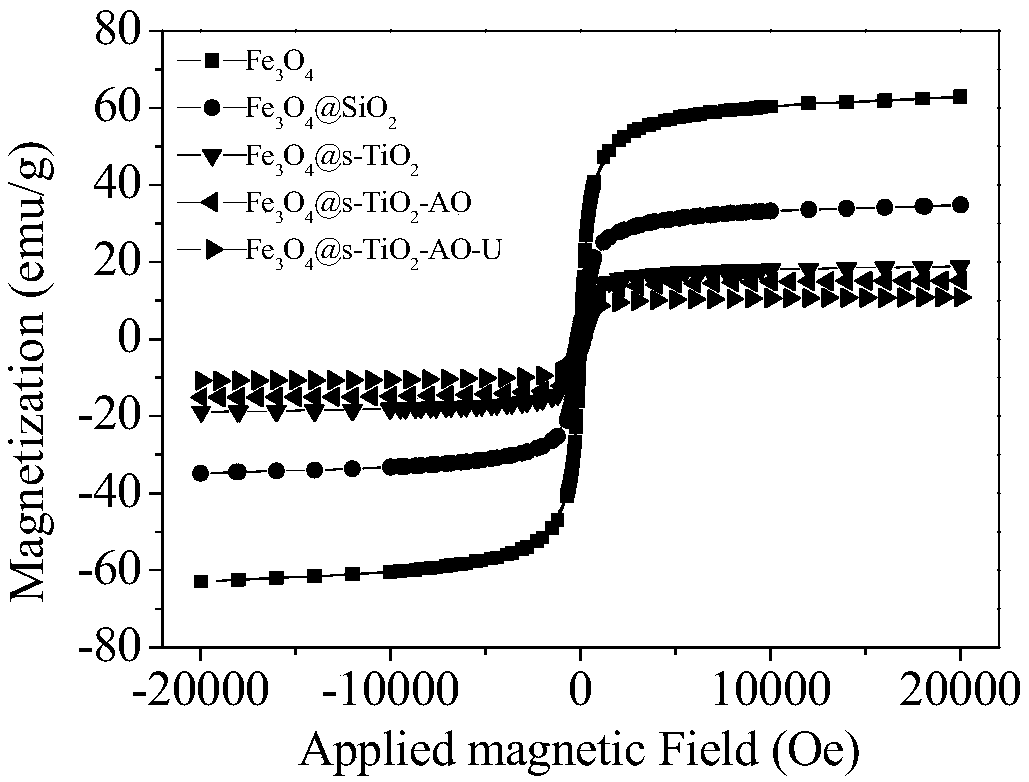
The invention discloses a magnetic nano functional material for extracting uranium from seawater, and a preparation method thereof. The preparation method includes the following steps: step 1, reacting ferric chloride hexahydrate with trisodium citrate dihydrate to obtain Fe3O4 particles; step 2, preparing Fe3O4@SiO2 particles through a compound reaction; step 3, dispersing Fe3O4@SiO2 in a mixed solution of ethanol and ammonium hydroxide, adding titanium isopropoxide, and obtaining Fe3O4@SiO2@TiO2 particles after reaction; step 4, dispersing Fe3O4@SiO2@TiO2 in an NaOH solution, and performingcalcination after reaction to obtain Fe3O4@s-TiO2 particles with developed surfaces; step 5, dispersing Fe3O4@s-TiO2 in acetic acid, adding a crosslinking agent, and carrying out an oil bath reactionto obtain cyanated Fe3O4@s-TiO2-CN particles; step 6, dispersing Fe3O4@s-TiO2-CN in a mixed solution of methanol and water, adding hydroxylamine hydrochloride, adjusting the pH of the solution to 7, and carrying out an oil bath reaction to obtain amidoximated Fe3O4@s-TiO2-AO particles. The Fe3O4@s-TiO2-AO material prepared by the preparation method has a developed surface, good magnetic properties, high stability, durability and bio-resistance and excellent adsorption selectivity for the uranium, and can be used for eliminating the uranium in uranium-containing water bodies and extracting theuranium from the seawater.
Owner:LANZHOU UNIVERSITY
Trichoderma aureoviride particle adsorbent for treating uranium-bearing wastewater as well as preparation method and application of absorbent
InactiveCN103949224ALow costEasy to prepareOther chemical processesMicroorganism based processesSterile waterSewage treatment
The invention relates to a trichoderma aureoviride particle adsorbent for treating uranium-bearing wastewater as well as a preparation method and application of the absorbent, and belongs to the technical field of production and manufacturing of a sewage treatment material. The adsorbent is prepared from trichoderma aureoviride fungus purely-cultured viable bacteria, corncob particles and an embedding agent and is 4mm-6mm in particle size; the preparation method comprises the following steps: culturing the trichoderma aureoviride bacteria and washing with sterile water to obtain conidium suspension liquid; washing, drying and crushing corncobs, and then sieving the particles of which the particle size is 1mm-2mm; and adsorbing, embedding, culturing and freeze-drying conidiums to obtain the trichoderma aureoviride particle adsorbent. The application is as follows: the trichoderma aureoviride particle adsorbent is added into a uranium-bearing water solution, and then the vibration and the adsorption are performed. The particle adsorbent prepared by adopting the method disclosed by the invention is low in cost, simple and convenient to operate, free of secondary pollution, liable to separate from the water solution, reusable, and capable of being used for effectively adsorbing and recycling uranium ions in the water solution.
Owner:EAST CHINA UNIV OF TECH
Nano composite adsorbent as well as preparation method and application thereof
ActiveCN109092258ASimple and fast manufacturing methodEasy to operateOther chemical processesRadioactive decontaminationSorbentDissolution
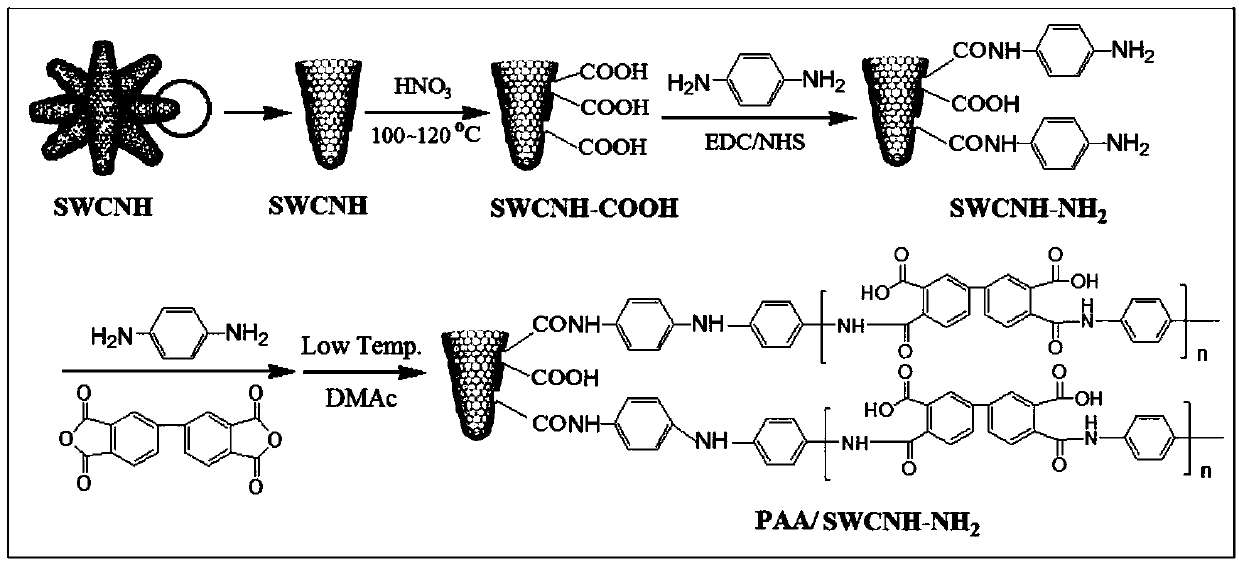
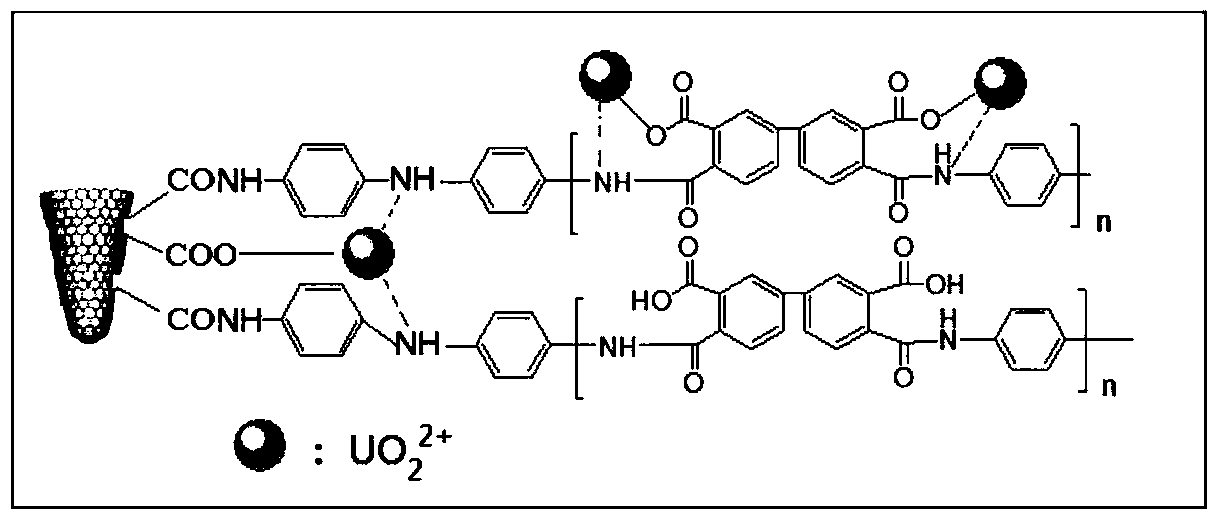
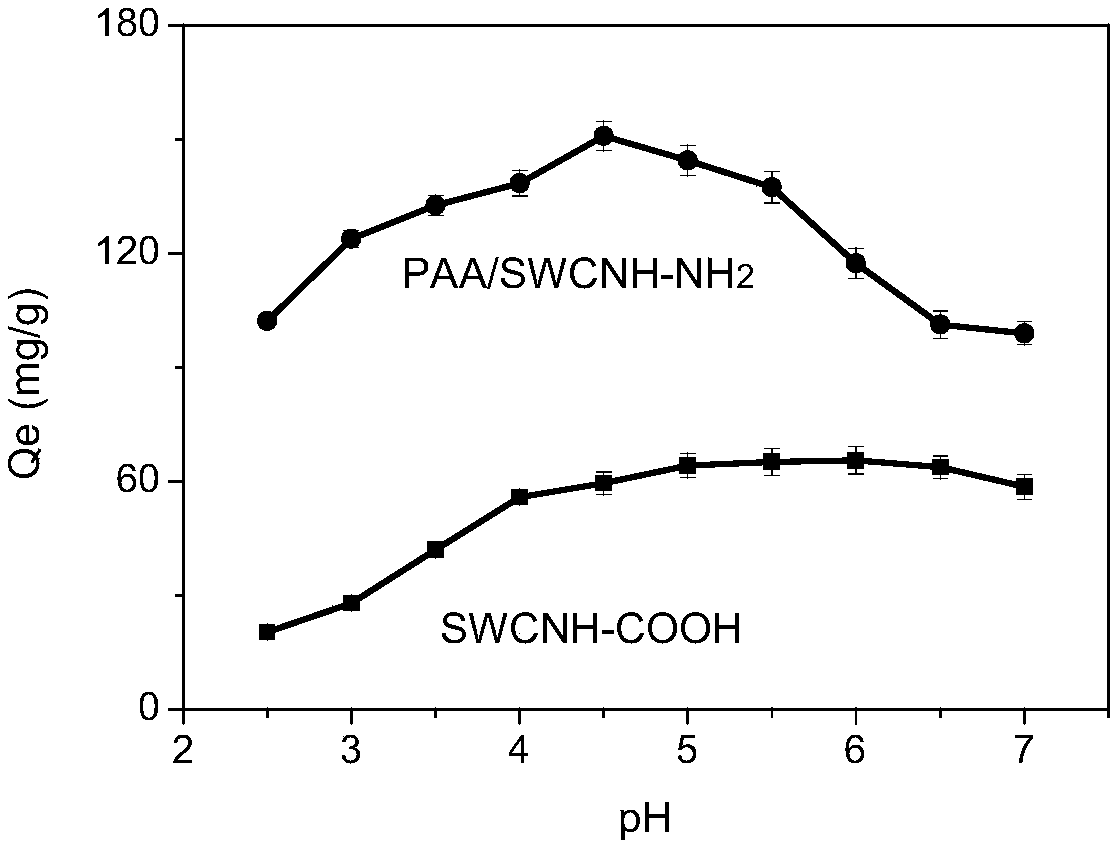
The invention provides a nano composite adsorbent as well as a preparation method and an application thereof. The preparation method comprises the following steps: dispersing SWCNH into a nitric acidsolution for modification to obtain carboxylated SWCNH; catalyzing carboxylated SWCNH and p-phenylenediamine as reaction raw materials by a catalyst under protection of nitrogen to obtain SWCNH-NH2 nanofiller; mixing and stirring 3,3',4,4'-biphenyltetracarboxylic dianhydride with anhydrous N,N-dimethylacetamide until complete dissolution to obtain a solution I; adding the SWCNH-NH2 nanofiller to the solution I, and performing stirring in an ice-water mixture environment at 0 DEG C to obtain a solution II; adding p-phenylenediamine to the solution II, continuing to stir at a low temperature toenable reactants to be fully reacted, and finally obtaining a viscous pale yellow polyamic acid solution which is uniformly dispersed. The prepared PAA / SWCNH-NH2 nanocomposite adsorbent has high adsorption capacity for uranyl ions in an aqueous solution, high adsorption speed, good reusable effect and strong selective adsorption capacity for uranium ions, and can effectively adsorb and recover uranyl ions from the aqueous solution.
Owner:EAST CHINA UNIV OF TECH
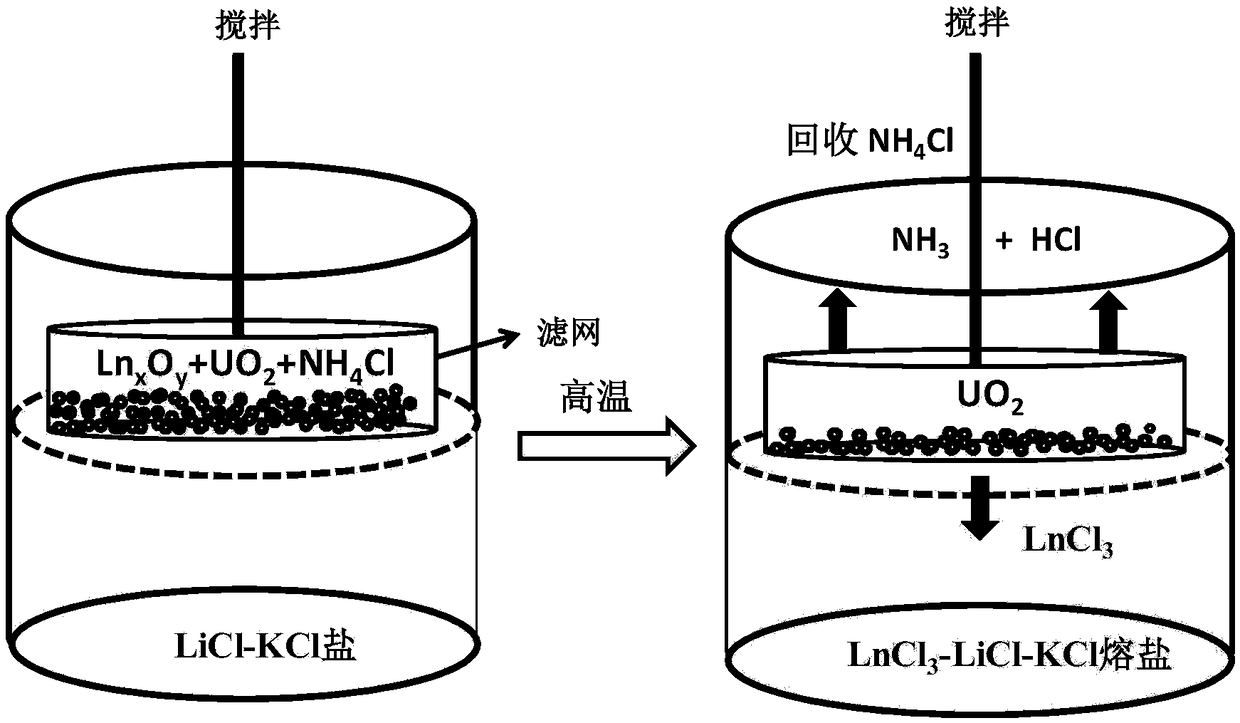
Application of ammonium chloride for separating uranium dioxide and lanthanide oxide
ActiveCN108364703ASolving insoluble puzzlesPracticalNuclear energy generationRecycling and recovery technologiesLanthanideMolten salt
The invention provides an application of ammonium chloride for separating uranium dioxide and lanthanide oxide, and provides a method for separating uranium dioxide and lanthanide oxide by using ammonium chloride. The method is characterized in that ammonium chloride is added in a reactant containing uranium dioxide and lanthanide oxide, the materials are contacted with chloride molten salt at thetemperature of 450-600 DEG C, the reactant and the chloride molten salt are controlled for isolating air, and the uranium dioxide and lanthanide oxide are separated. The application of the ammonium chloride in separation of dioxide solves the difficult dissolving problem of uranium dioxide in molten salt, and the reaction condition is controlled to separate the uranium dioxide and lanthanide oxide. The method can directly realize UO2 dissolution in a chloride molten salt system, and can be carried out in air during a dissolving process, other deposition is not formed, other impurity is not introduced, strongly corrosive gas is not employed, and a whole process has the advantages of simple operation, mild reaction condition, and strong practicality.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACADEMY OF SCI
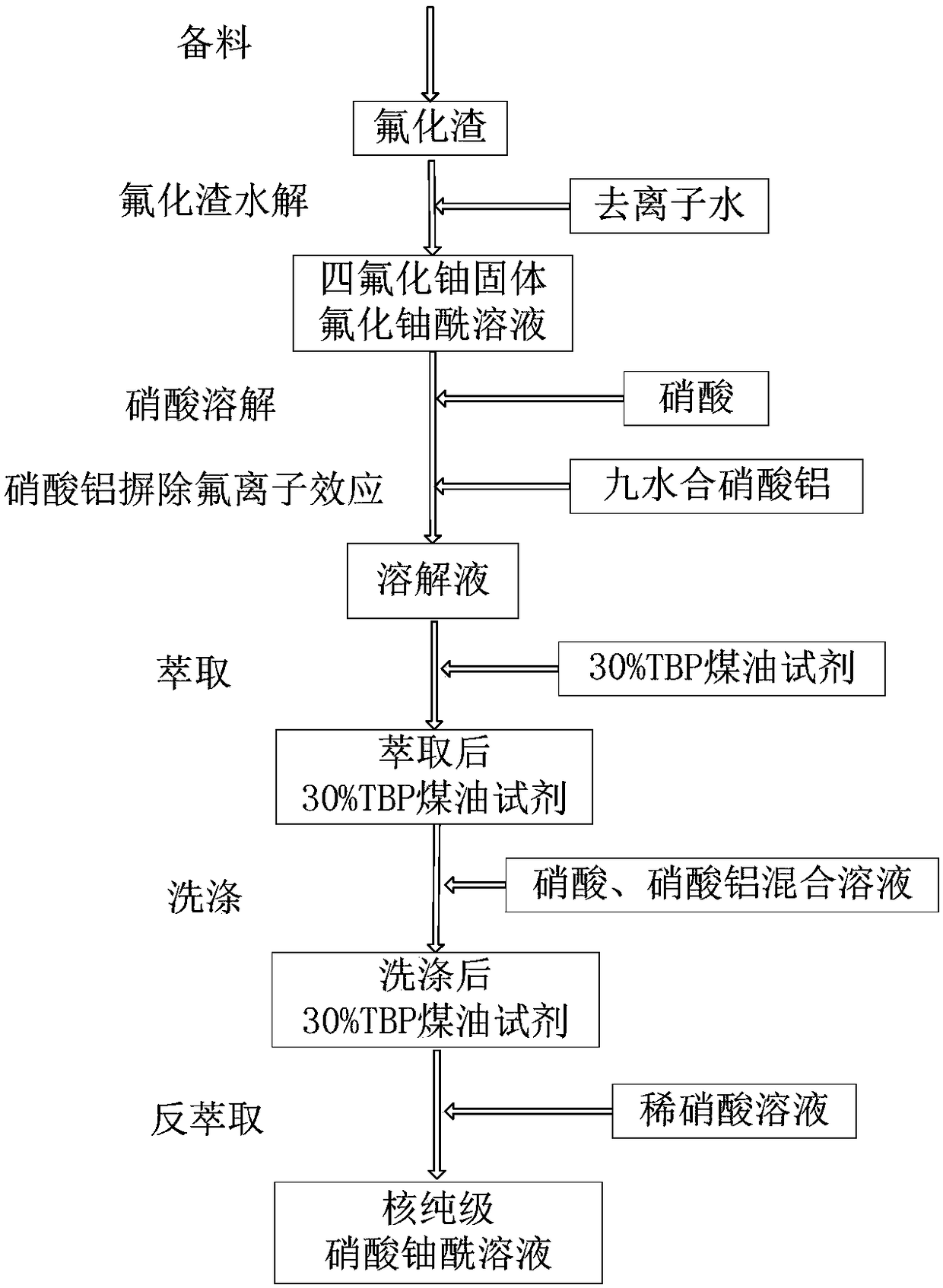
Extraction purification method for recycling uranium from fluoridation ash residues
InactiveCN108165747ASimple structureEasy to operateProcess efficiency improvementMetal impuritiesDissolution
The invention relates to the technical field of uranium conversion and particularly discloses an extraction purification method for recycling uranium from fluoridation ash residues. The extraction purification method comprises the following steps of 1, preparing materials; 2, conducting hydrolysis on the fluoridation residues; 3, conducting dissolution through nitric acid, and eliminating the fluorinion effect through aluminum nitrate; and 4, conducting extraction, washing and reextraction, so that a nuclear-purity-grade uranyl nitrate solution is obtained. According to the extraction purification method, special properties of a fluorination residue material are combined, and a process route which can be used for recycling the uranium from the fluoridation residues is developed; the process route is novel in concept, simple in procedure and feasible; by means of the method, metal impurity elements in the fluoridation residues can be effectively removed, and the nuclear-purity-grade uranyl nitrate solution is obtained; and the nuclear-purity-grade uranyl nitrate solution can be directly used as a uranium purification conversion process raw material.
Owner:THE 404 COMPANY LIMITED CHINA NAT NUCLEAR
Polyamidoxime-based chelating resin for extracting uranium from seawater and preparation method thereof
ActiveCN111171208ASimple and fast manufacturing methodEasy to operateOther chemical processesSeawater treatmentPolymer adsorbentChelating resin
The invention relates to polyamidoxime-based chelating resin for extracting uranium from seawater and a preparation method thereof, and is characterized in that the polymer chelating resin is preparedby adopting a solvothermal method, and a chelating group is amidoxime; amidoxime styrene and ethylene glycol dimethacrylate are copolymerized under a solvothermal condition, and high-concentration amidoxime is immobilized on a polymer skeleton, so that the amidoxime chelating resin adsorbing material is prepared, and the amidoxime chelating resin adsorbing material is applied as a polymer adsorbent for separating and enriching uranium from seawater. The preparation route of the synthesized polyamidoxime-based chelating resin is simple and easy to operate. Meanwhile, the adsorbent has the advantages of being high in adsorption capacity, large in adsorption capacity, high in adsorption speed and the like when used for separating and enriching uranyl ions in seawater, is easy to separate from an aqueous solution, and can effectively adsorb and recover uranyl ions in the seawater solution.
Owner:EAST CHINA UNIV OF TECH
Biological ferrous sulfide and uranium-polluted underground water treatment permeation reaction wall based on same
InactiveCN105819576AHigh yieldSimple and fast operationWater contaminantsRadioactive decontaminationPermeationPollution
The invention discloses a biological ferrous sulfide composite material which comprises ferrous sulfide and synthetic bacteria bonded to the ferrous sulfide .The ratio of the number of microorganisms of the synthetic bacteria to the ferrous sulfate of unit mass is 3.3*107-5*107 cfu / mg .The synthetic bacteria mainly comprise Desulfovibrio and Clostridium .The invention further discloses a uranium-polluted underground water treatment permeation reaction wall based on the biological ferrous sulfide .The uranium-polluted underground water treatment permeation reaction wall comprises a reaction well and a reaction medium .The reaction well is arranged in soil containing uranium-polluted underground water and located on an underground water flowing path, and the top of the reaction well is flush with the ground and provided with an upward first opening .The reaction medium is arranged in the reaction well and is the biological ferrous sulfide composite material .The prepared material and the device have high removal rate for uranium in water, secondary pollution is avoided, regenerability is good, and the requirements of current underground water uranium-polluted permeation reaction wall materials are met.
Owner:NANHUA UNIV
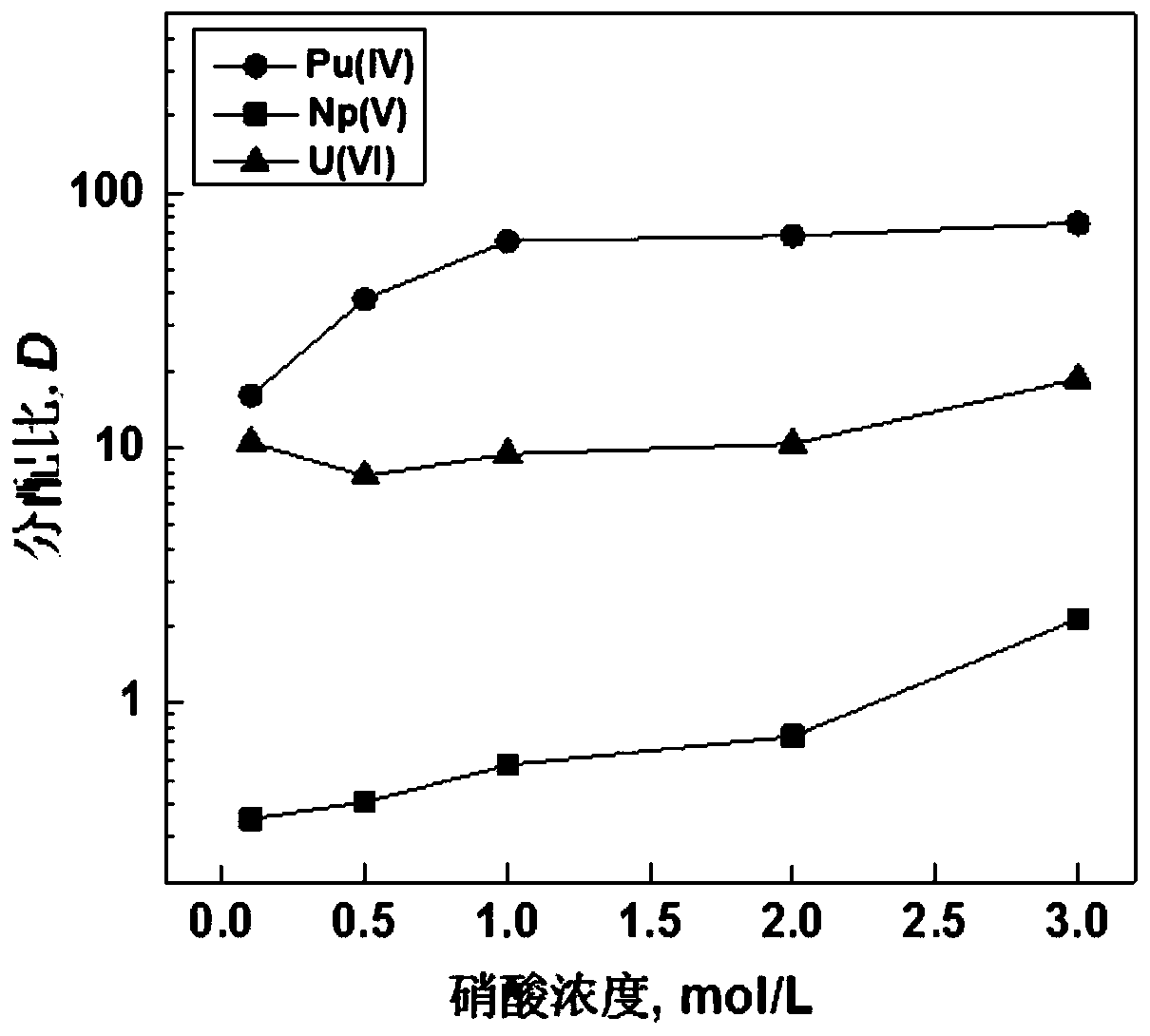
Tetravalent plutonium ion extraction method
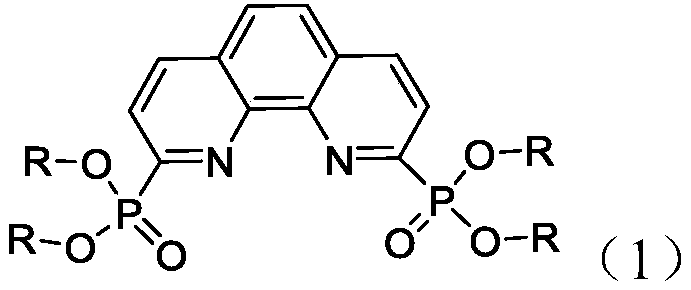
ActiveCN110894578AImprove extraction abilityFast extractionGroup 5/15 element organic compoundsProcess efficiency improvementPhenanthrolineDiluent
The invention relates to the technical field of nuclear fuel circulation and waste liquid treatment, and discloses a tetravalent plutonium ion extraction method, which comprises steps of: mixing a phenanthroline derivative represented by a formula (1) and a diluent to form an organic phase, and extracting tetravalent plutonium ions from an aqueous phase. The aqueous phase is an acidic aqueous solution containing tetravalent plutonium ions. The phenanthroline derivative is used as an extracting agent, tetravalent plutonium is selectively extracted from the aqueous phase containing various actinide ions, the extraction capacity is high, the extraction speed is high, particularly, the tetravalent plutonium extraction and separation efficiency is very high from a nitric acid aqueous solution containing pentavalent neptunium and hexavalent uranium, the extraction rate can reach 99% at most, and the method has a good application prospect in the field of nuclear industry waste liquid treatment.
Owner:ZHEJIANG UNIV
Preparation method for synthesizing MIL-53(Al)-AO2 on basis of metal-organic framework of amidoxime
InactiveCN108484929AHigh selectivityImprove adsorption capacityOther chemical processesRadioactive contaminantsFiltrationAcrylonitrile
The invention discloses a preparation method for synthesizing MIL-53(Al)-AO2 on the basis of a metal-organic framework of amidoxime. The preparation method comprises the following steps: 1, dissolving2-aminaterephthalic acid and Al(NO3)3.9H2O into DMF (Dimethyl Formamide) and deionized water to prepare a MIL-53(Al)-NH2 mixture A1; 2, washing impurities in the A1 with DMF and acrylonitrile so as to obtain a solid product A2; 3, adding the A2 into acrylonitrile and Al Cl3 to be fully dissolved so as to obtain a middle product A3; 4, distributing the A3 into a mixed solution of ultrapure water and ethanol, adding K2CO3 and NH2OH.HCl, and reacting to obtain a mixture A4; 5, washing the A4 with ethanol and ultrapure water, performing suction filtration, separating, and performing vacuum drying, so as to obtain the MIL-53(Al)-AO2. The preparation method disclosed by the invention has the beneficial effects that a preparation method of the MIL-53(Al)-AO2 with advantages of being high in yield, simple in equipment and easy to operate and control is provided; a high-efficiency material is provided for extracting uranium from uranium-bearing water.
Owner:TIANJIN CHENGJIAN UNIV
Method for removing organic matter in uranium-bearing alkaline leaching solution
InactiveCN107805712ANo signs of poisoningDecreased saturated adsorption capacityPregnant leach solutionContact time
The invention belongs to the technical field of organic matter separation and particularly relates to a method for removing organic matter in a uranium-bearing alkaline leaching solution. The method comprises the following steps that (1) the organic matter is removed through weakly alkaline adsorption resin, specifically, the weakly alkaline acrylic skeleton adsorption resin is added into an ion exchanging column, the uranium-bearing alkaline leaching solution is selected so as to conduct a dynamic adsorption test on the organic matter in the uranium-bearing alkaline leaching solution; (2) drip washing of the weakly alkaline adsorption resin is conducted, specifically, before drip washing, the column is washed through a sodium carbonate solution with the mass concentration being 5g / L; and(3) uranium is recovered through strongly alkaline anion exchanging resin, specifically, the strongly alkaline anion exchanging resin is added into the ion exchanging column, the uranium-bearing alkaline leaching solution treated in the step (1) is selected, and uranium in the uranium-bearing alkaline leaching solution is subjected to dynamic adsorption, wherein the contact time is 5-15 min, and the penetration concentration is determined to be 5 mg / L. Through the method, the problem of organic matter poisoning in the technique of ion exchanging for uranium extraction can be effectively solved, uranium is not adsorbed while the organic matter is adsorbed and removed, the uranium separating efficiency is improved, and technological support is provided for an alkaline method uranium extraction technique.
Owner:BEIJING RESEARCH INSTITUTE OF CHEMICAL ENGINEERING AND METALLURGY
Method for absorbing uranium with uranium template ion imprinted ploy(N-isopropyl acrylamide)/chitosan interpenetrating polymer network hydrogel
The invention relates to a method for absorbing uranium with a uranium template ion imprinted ploy(N-isopropyl acrylamide) / chitosan interpenetrating polymer network hydrogel. The method comprises the following steps: with chitosan as a monomer, dissolving chitosan in acetic acid, adding a high-concentration uranium solution under certain conditions and allowing chitosan to adsorb uranium ions; then adding N-isopropyl acrylamide (NIPAAm) monomer and simultaneously adding an N,N '-methylenebisacrylamide (MBA) cross-linking agent, an ammonium persulfate (APS) initiator and an N,N,N ',N '-tetramethyl ethylenediamine (TEMED) promoter so as to prepare the U(VI) imprinted PNIPAAm / CS interpenetrating polymer network hydrogel; and researching adsorptivity of U(VI). Compared with traditional gel, the hydrogel provided by the invention has the characteristics of good adsorption selectivity, a great adsorption surface area, high uranium adsorption capacity, etc.
Owner:EAST CHINA UNIV OF TECH
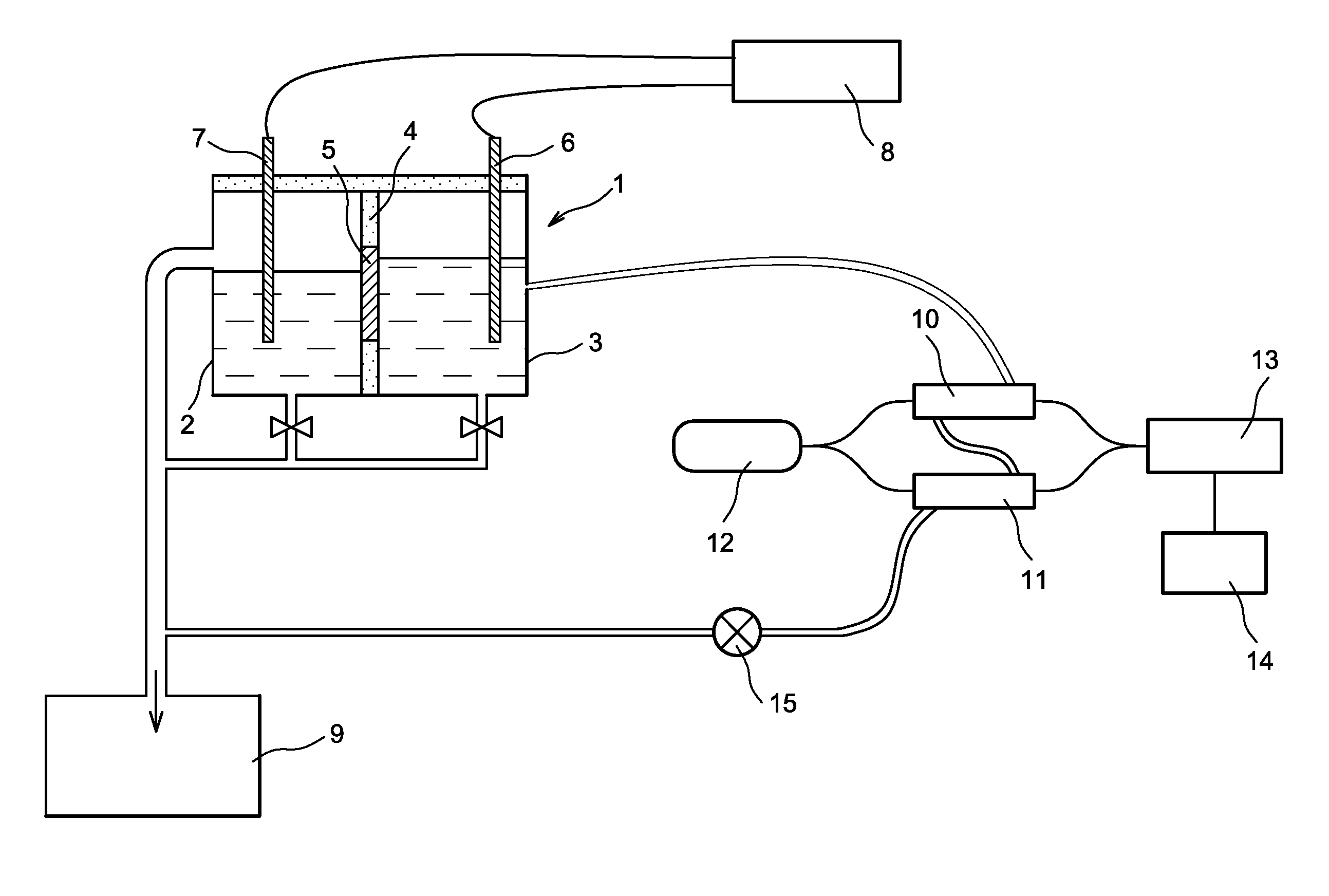
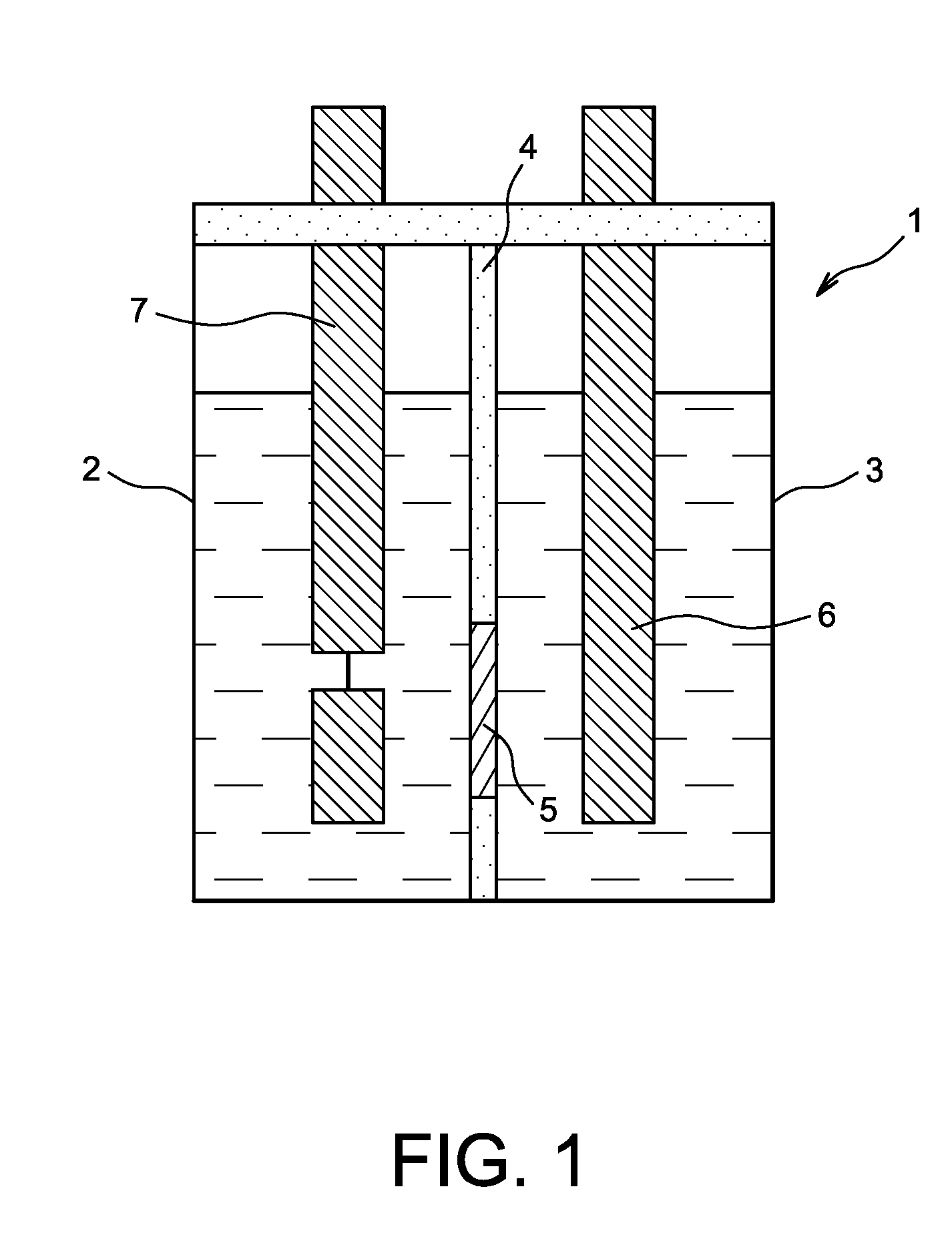
Super oxidation, coagulation and flocculation system for treatment of water and wastewater
ActiveUS8597507B2Prevent escapeLiquid degasificationMixing methodsChemical treatmentPotassium permanganate
The super oxidation, coagulation and flocculation system is applicable to water and wastewater chemical treatment in which oxidation, coagulation and flocculation processes take place in a single vessel.The apparatus and process can be used for treatment of domestic and industrial water and wastewater for removal of organic and inorganic contaminants.The oxidation process can be provided by several oxidizing chemicals such as ozone, hydrogen peroxide, potassium permanganate, chlorine and Ultra Violet Lights, and the coagulation process can be accomplished with several coagulants such as aluminum sulphide, ferric chloride, ferric sulphate.Selection of the chemicals depends on the quality of the water or wastewater to be treated and the treated water quality required, and it covers a wide range of impurities to be removed and specifically difficult to oxidize hydrocarbons and chlorinated organics, and iron, manganese, uranium, arsenic, cyanide, hydrogen sulphide.The apparatus and the process are economical and have a small installation footprint as several processes take place in a single vessel.
Owner:KORZENIOWSKI JAN A
Method for preparing hollow UO2 nanospheres by ammonium uranyl carbonate solution irradiation
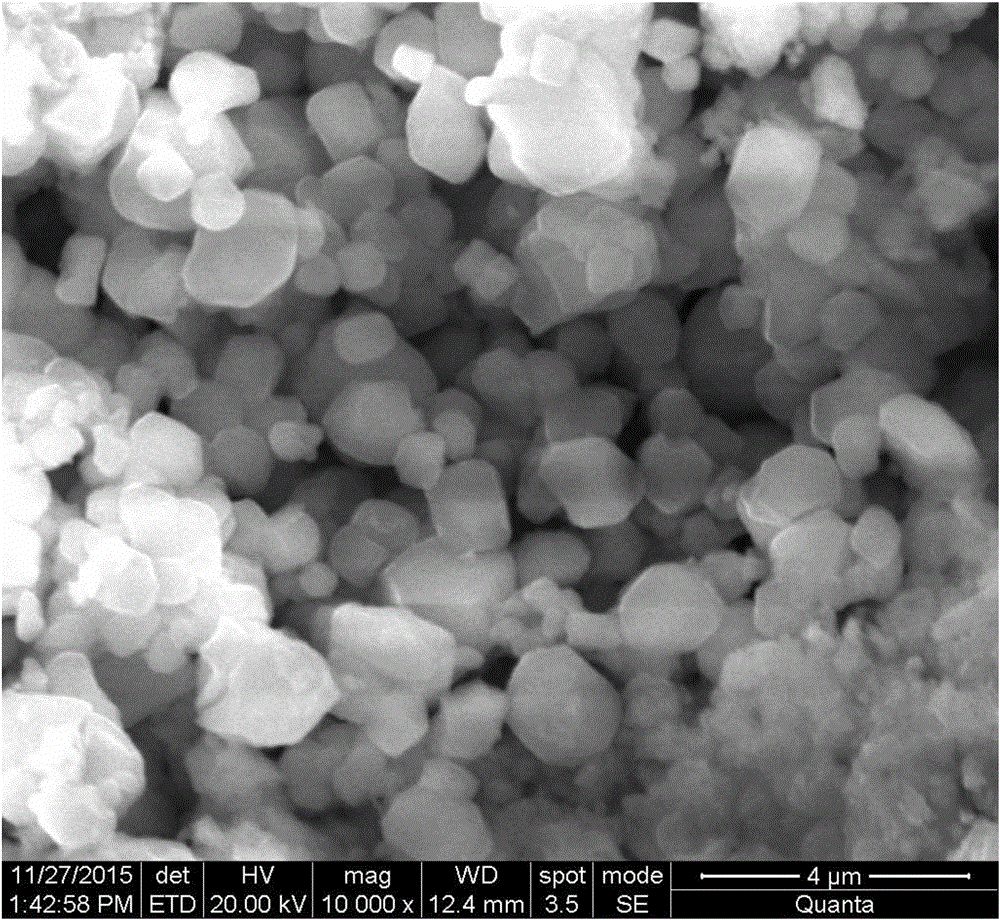
The invention discloses a method for preparing hollow UO2 nanospheres by ammonium uranyl carbonate solution irradiation. The method includes: preparing low-concentration UO2(CO3)3<4-> alkaline solution containing a free radical removing agent; adopting electron beam or gamma-ray irradiation to obtain the hollow UO2 nanospheres, different in diameter, wall thickness and cavity diameter, formed by self assembly of nanoparticles through control of conditions such as absorbed dose and dose rate. A uranium oxide hollow nanostructure which is prepared for the first time is conducive to researches of application of uranium oxide nanoparticles to fields of nuclear fuel, catalysis and the like.
Owner:PEKING UNIV
Method for preparing aqueous solution of neutral negative oxygen ions
PendingCN110272064AImprove sustained releaseResolve Material EffectsOther chemical processesRadium compoundsThermal insulationThorium nitrate
The invention discloses a method for preparing an aqueous solution of neutral negative oxygen ions. The method comprises the steps: performing a reaction on monazite concentrate and a sodium hydroxide aqueous solution of 45-55% at a temperature of 130-150 DEG C for 4.5-5.5 h so as to obtain a reactant with a mass ratio of alkaline to ore at (1:1)-(1:1.5), then diluting the reactant by using hot water of 80-100 DEG C, performing thermal insulation aging at 60-80 DEG C, performing separation so as to obtain a monazite alkali-soluble cake, then dissolving the alkali-soluble cake completely by using nitric acid, then extracting uranium thorium by using tributyl phosphate of 25-35%, performing reverse extraction by using pure water after introduction of uranium thorium into an organic phase so as to obtain a strip liquid of uranium thorium, performing concentration, then extracting uranium by using tributyl phosphate of 5.5-6.5%, extracting thorium by using a TBP kerosene liquid of 35-45%, and then performing reverse extraction, concentration and crystallization so as to obtain thorium nitrate. Through the colorless transparent neutral aqueous solution of the negative oxygen ions, influence and damage of an original acidic solution on the material of an attached substance are avoided.
Owner:广州曜科环保科技有限公司
Method for separating uranium and thorium and reagent used for separation
InactiveCN103451426AAvoid adjustmentAvoid Co-Extraction Reagent InsufficiencyIonic liquidMethyl palmoxirate
The invention discloses a method for separating uranium and thorium and an extraction agent used for separation. According to the method, uranium and thorium nitrate mixed aqueous solution is placed in imidazolyl ionic liquid diluent in which the reagent is dissolved or n-amyl alcohol diluent in which the reagent is dissolved, thorium is extracted, and uranium stays in an aqueous phase. The extraction agent used for separation is1-methylimidazole showed in a first formula or 2-methylimidazole showed in a second formula. The method and the reagent have the advantages that the extraction agent is cheap and can be obtained easily, an extraction system is simple, extraction efficiency is high, the number of used extraction agents is small, and specificity is strong.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com