Recovering method for uranium and fluorine in uranium hexafluoride alkali absorption solution waste liquid
A technology of uranium hexafluoride alkali and recovery method, which is applied in the fields of hydrometallurgy and nuclear fuel recovery, which can solve the problems of cumbersome process, increased waste liquid discharge, and high cost, and achieve the effect of simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
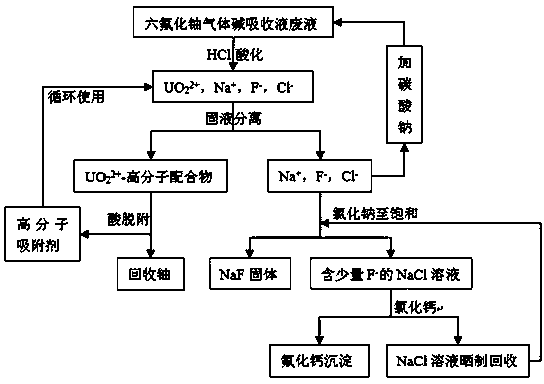
[0039] Method for recovering uranium and fluorine in uranium hexafluoride alkali absorption liquid waste liquid, the waste liquid contains UO 2 2+ up to 48 mg / L, F after 5 cycles - up to 9 g / L. Take 1 L of the waste liquid as the research object, the specific reaction route and operation steps are as follows:
[0040] Step 1, acidification of uranium hexafluoride alkali absorption liquid waste liquid
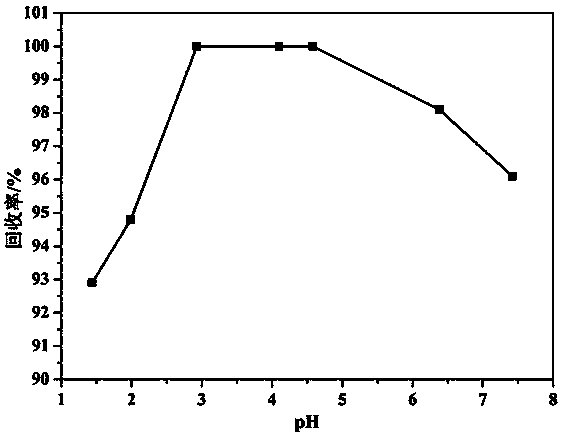
[0041] Add 1 M hydrochloric acid dropwise to the waste liquid of uranium hexafluoride gas alkali absorption liquid, adjust pH = 4.5, and obtain 2 2+ 、Na + , F - with Cl - The solution;
[0042] Step two, UO 2 2+ recycling
[0043] a. UO 2 2+ Adsorption
[0044] Add 0.2 g of polymer adsorbent polystyrene-cyclohexylaminomaleic acid to the solution obtained in step 1, and stir for 10 min to make the UO in the solution 2 2+ Adsorption equilibrium, filtration and separation to obtain UO 2 2+ -Polystyrene-cyclohexylaminomaleic acid complex solid and containing Na + ...
Embodiment 2
[0051] Method for recovering uranium and fluorine in the waste liquid of uranium hexafluoride gas alkali absorption liquid, the waste liquid contains UO 2 2+ up to 130 mg / L, F after 5 cycles - up to 8 g / L. Take 1 L of this waste liquid as the research object:
[0052] Step 1, acidification of uranium hexafluoride alkali absorption liquid waste liquid
[0053] Add 2 M hydrochloric acid dropwise to the waste liquid of uranium hexafluoride gas alkali absorption liquid, adjust the pH = 3.8, and obtain 2 2+ 、Na + , F - with Cl - The solution;
[0054] Step two, UO 2 2+ recycling
[0055] a. UO 2 2+ Adsorption
[0056] Add 0.5 g of styrene-cyclohexylaminomaleic acid to the solution obtained in step 1, and stir for 15 min to make the UO in the solution 2 2+ Adsorption equilibrium, filtration and separation to obtain UO 2 2+ -Polystyrene-cyclohexylaminomaleic acid complex solid and containing Na + , F - with Cl - solution, the remaining amount of uranium in the so...
Embodiment 3
[0063] Method for recovering uranium and fluorine in the waste liquid of uranium hexafluoride gas alkali absorption liquid, the waste liquid contains UO 2 2+ up to 238 mg / L, F after 5 cycles - up to 10 g / L. Take 1 L of the waste liquid as the research object, the specific reaction route and operation steps are as follows:
[0064] Step 1, acidification of uranium hexafluoride alkali absorption liquid waste liquid
[0065] Add 2 M hydrochloric acid dropwise to the waste liquid of uranium hexafluoride gas alkali absorption liquid, adjust the pH = 3.5, and obtain 2 2+ 、Na + , F - with Cl - The solution;
[0066] Step two, UO 2 2+ recycling
[0067] a. UO 2 2+ Adsorption
[0068] Add 1 g of polymer adsorbent polystyrene-cyclohexylaminomaleic acid to the solution obtained in step 1, and stir for 15 min to make the UO in the solution 2 2+ Adsorption equilibrium, filtration and separation to obtain UO 2 2+ -Polystyrene-cyclohexylaminomaleic acid complex solid and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com