Extraction purification method for recycling uranium from fluoridation ash residues
A technology of fluorination reaction and purification method, which is applied in the field of uranium conversion, and can solve problems such as unsystematic research on uranium recovery technology in fluoride slag, ash dumping, difficulty in crushing, and small production capacity of UF6 , achieving significant economic and environmental benefits, easy industrial application, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
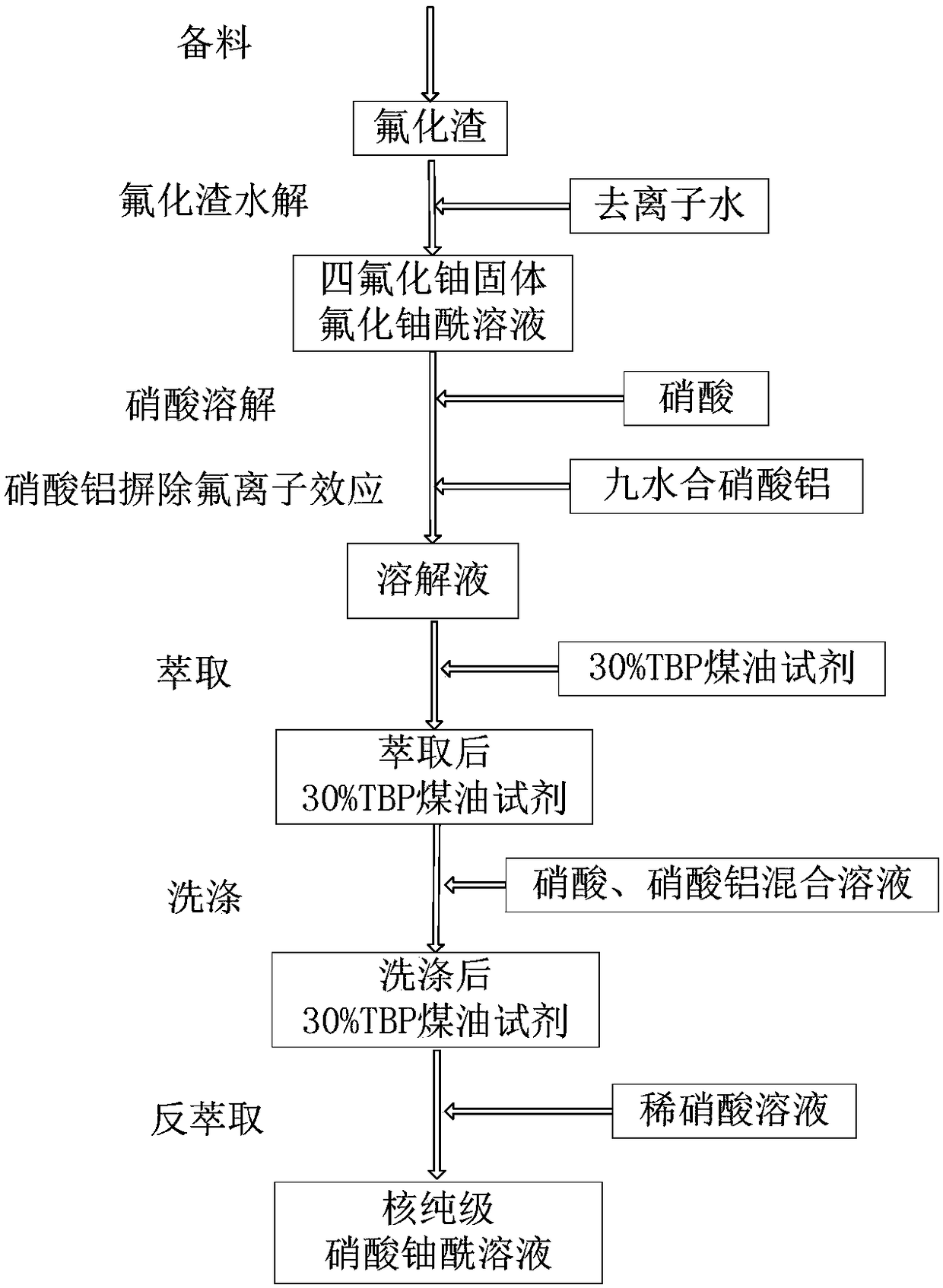
[0051] Step 1: Prepare materials;
[0052] Take out the fluoride slag material from the fluoride slag tank;
[0053] Step 2: hydrolysis of fluoride slag;
[0054] Place the taken out fluoride slag in the hydrolysis reactor, add deionized water heated to 70°C according to the mass ratio of fluoride slag to deionized water of 1:1, start the stirring device of the hydrolyzer, and deionize the fluoride slag Hydrolysis, during the hydrolysis process, control the reaction temperature to keep at 70°C, and react for 45 minutes;
[0055] Step 3: Nitric acid dissolution, aluminum nitrate eliminates the effect of fluoride ions;
[0056] According to the amount that the mass fraction of 65% nitric acid and the mass ratio of fluoride slag is 1:1, add 65% nitric acid to the hydrolyzed fluoride slag solution, react for 4 hours, control the reaction temperature to be 70° C. The uranium fluoride solid material is completely dissolved;
[0057] According to the ratio of the molar amount of ...
Embodiment 2
[0063] Step 1: Prepare materials;
[0064] Take out the fluoride slag material from the fluoride slag tank;
[0065] Step 2: hydrolysis of fluoride slag;
[0066] Place the taken out fluoride slag in the hydrolysis reactor, add deionized water heated to 80°C according to the mass ratio of fluoride slag to deionized water of 1:2, start the stirring device of the hydrolyzer, and deionize the fluoride slag Hydrolysis, during the hydrolysis process, control the reaction temperature to keep at 80°C, and react for 60 minutes;
[0067] Step 3: Nitric acid dissolution, aluminum nitrate eliminates the effect of fluoride ions;
[0068] According to the mass ratio of 65% nitric acid and fluoride slag mass ratio of 1.5:1, add 65% nitric acid in mass fraction to the hydrolyzed fluoride slag solution, react for 6 hours, and control the reaction temperature to be 75° C. The solid material of uranium fluoride is completely dissolved. According to the ratio of the molar amount of aluminum ...
Embodiment 3
[0074] Step 1: Prepare materials;
[0075] Take out the fluoride slag material from the fluoride slag tank;
[0076] Step 2: hydrolysis of fluoride slag;
[0077] Place the taken out fluoride slag in the hydrolysis reactor, add deionized water heated up to 75°C according to the mass ratio of fluoride slag to deionized water of 1:1.5, start the stirring device of the hydrolyzer, and deionize the fluoride slag Hydrolysis, during the hydrolysis process, control the reaction temperature to keep at 75°C, and react for 50 minutes;
[0078] Step 3: Nitric acid dissolution, aluminum nitrate eliminates the effect of fluoride ions;
[0079] According to the amount that the mass fraction of 65% nitric acid and the mass ratio of fluoridated slag is 1.3:1, add the nitric acid of mass fraction 65% to the hydrolyzed fluorided slag solution, react for 5h, control the reaction temperature to be 73 DEG C, make the hydrolyzed four The solid material of uranium fluoride is completely dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com