Preparation method of porous covalent organic framework and application of porous covalent organic framework in uranyl ion capture
A technology of covalent organic framework and uranyl ion, which is applied to the preparation of porous covalent organic framework and the application field of capturing uranyl ions, can solve the problems of poor hydrophilicity, no application yet, poor visible light absorption ability, etc. To achieve the effect of increasing adsorption capacity, promoting adequate exposure, and improving electron transfer and mass transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
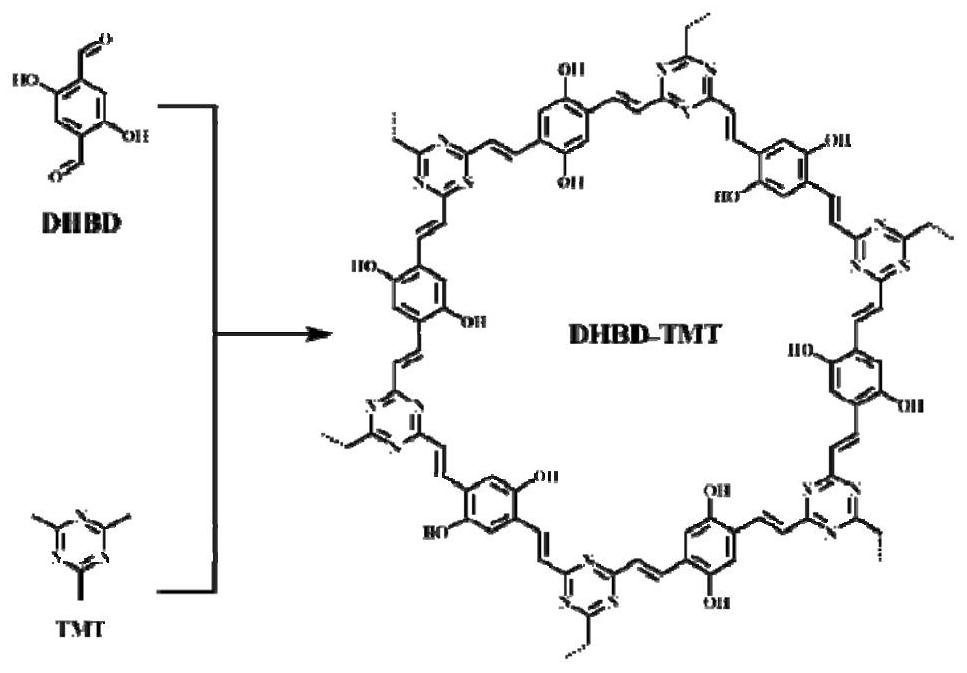
[0030] Example 1: A preparation method of a porous covalent organic framework
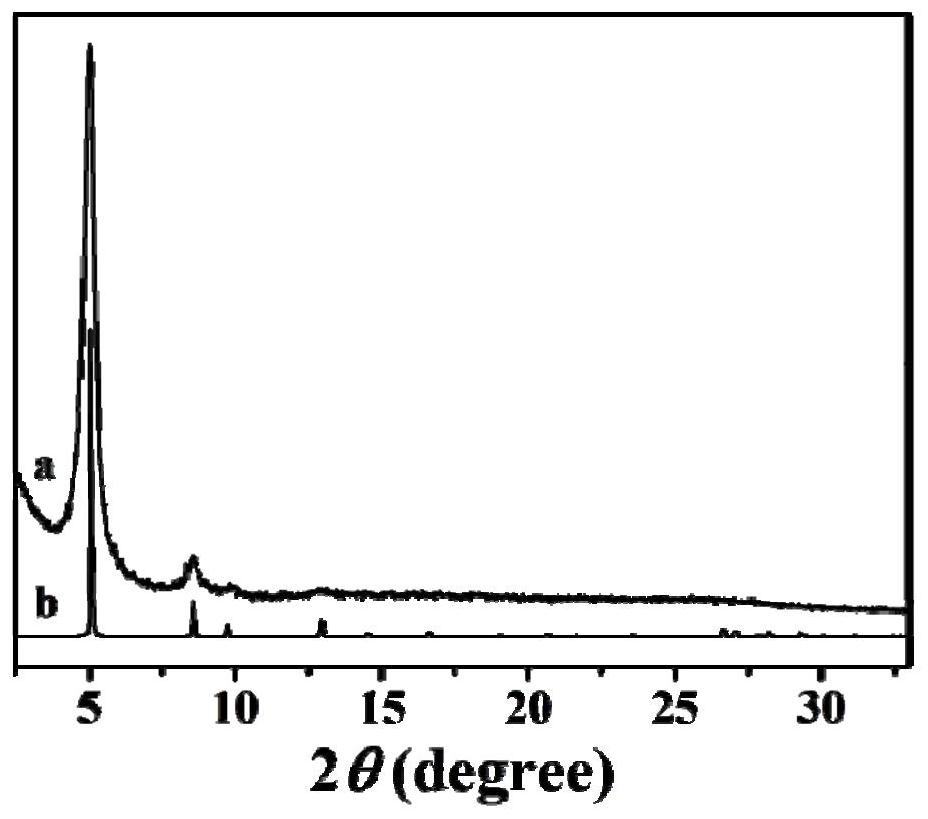
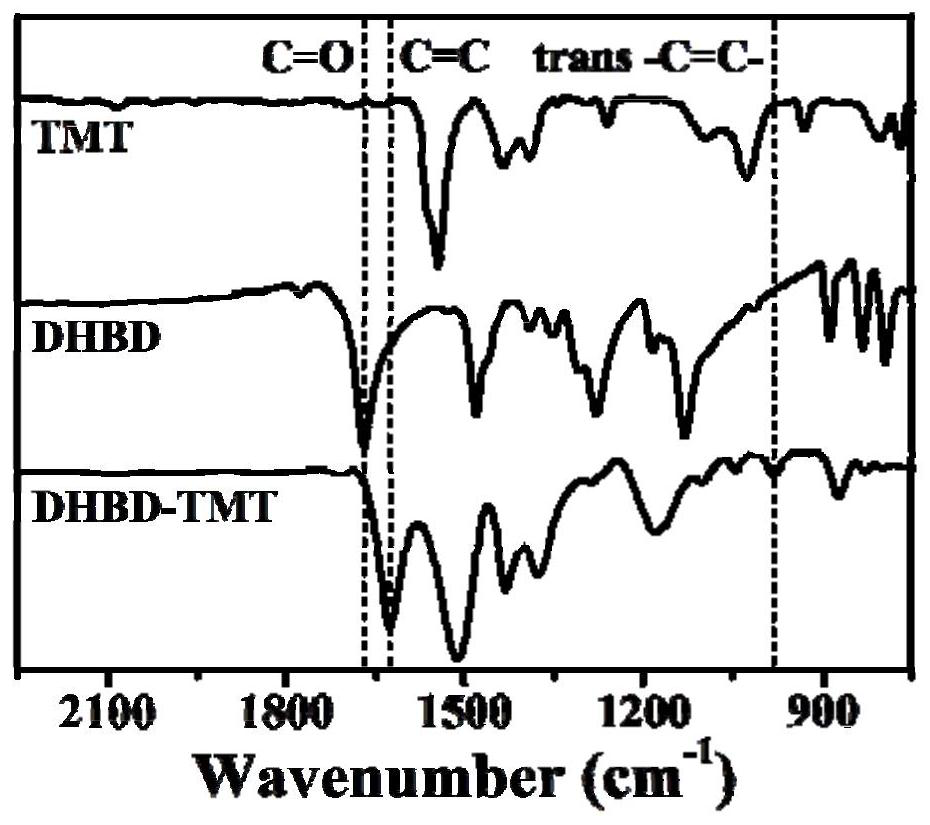
[0031] 12.5mg 2,5-dihydroxy-1,4-benzenedicarbaldehyde (DHBD), 6.2mg 2,4,6-trimethyl-1,3,5-triazine (TMT), 0.45mL mesitylene , 0.45mL 1,4-dioxane, 0.20mL trifluoroacetic acid and 0.025mL acetonitrile were added to a 10mL quartz tube, the quartz tube was quickly frozen in a liquid nitrogen bath, degassed and flame-sealed through three freeze-thaw cycles, The reaction mixture was ultrasonically treated to obtain a homogeneous solution; then, the reaction mixture was placed in an oven at 150°C, and stood for 3 days. After cooling to room temperature, the solid product was collected, washed three times with acetone and methanol, and washed with 0.1 mol / L N 4 Aqueous methanol solution of OH (50wt%) was neutralized to pH 7.0 to remove residual trifluoroacetic acid in the reaction, and then vacuum-dried at 90° C. for 12 hours to obtain olefin-bonded DHBD-TMT in the form of black powder. figure 1 It is a...
Embodiment 2
[0035] The photoelectric properties of DHBD-TMT were studied by UV-Vis absorption spectroscopy and Mott-Schottky spectroscopy. Figure 5 is the UV-Vis absorption spectrum and Mott-Schottky diagram of DHBD-TMT. Depend on Figure 5 a It can be seen that DHBD-TMT can effectively absorb light in the ultraviolet and visible light regions, and DHBD-TMT has a broad light absorption band, which provides the possibility for photocatalytic U(VI) under visible light irradiation. Depend on Figure 5 b It can be seen that the curve in the Mott-Schottky spectrum has a positive slope, indicating that DHBD-TMT is a typical n-type semiconductor, in which photogenerated electrons are the main carriers, and the height of the plane with unsubstituted vinylidene bonds π-conjugated skeletons. According to the x-intercept of the Mott-Schottky spectrum, the DHBD-TMT flat band potential (E fb ) is fitted to be -1.29V vs. NHE (standard hydrogen electrode), which is much more negative than the reduc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com