DNA vaccine capable of simultaneously expressing FAdV-4 spike protein 1 and spike protein 2 genes as well as construction method and application of DNA vaccine
A DNA vaccine, spike protein technology, applied in the field of biomedicine, can solve the problems of no vaccine, economic loss, etc., and achieve the effect of improving the effect of immune prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for constructing a DNA vaccine that simultaneously expresses FAdV-4 spike protein 1 and spike protein 2 genes, comprising the steps of:
[0031] 1. Design the specific primers P1 and P2 of FAdV-4 spike protein 1 and the specific primers P3 and P4 of spike protein 2, whose sequences are as follows:
[0032] P1: 5'AATTAAGCTTATGTCGGCCCTAATCGCCTCCGCAGCCG 3' (shown in SEQ ID No.2);
[0033] P2: 5'CCCGCCTGCTTAAGCAGGCTAAAGTTGGTCGCGCCGCTGCCGGGGCCCGGAGCATT3' (shown in SEQ ID No.3);
[0034] P3: 5'GCTTAAGCAGGCGGGCGATGTGGAAGAAAACCCGGGCCCGATGCTCCGGGCCCCT 3' (as shown in SEQID No.4);
[0035] P4: 5'TAAAGCGGCCGCTTACGGGAGGGAGGCCGCTGGACAGCTG 3' (shown in SEQ ID No. 5).
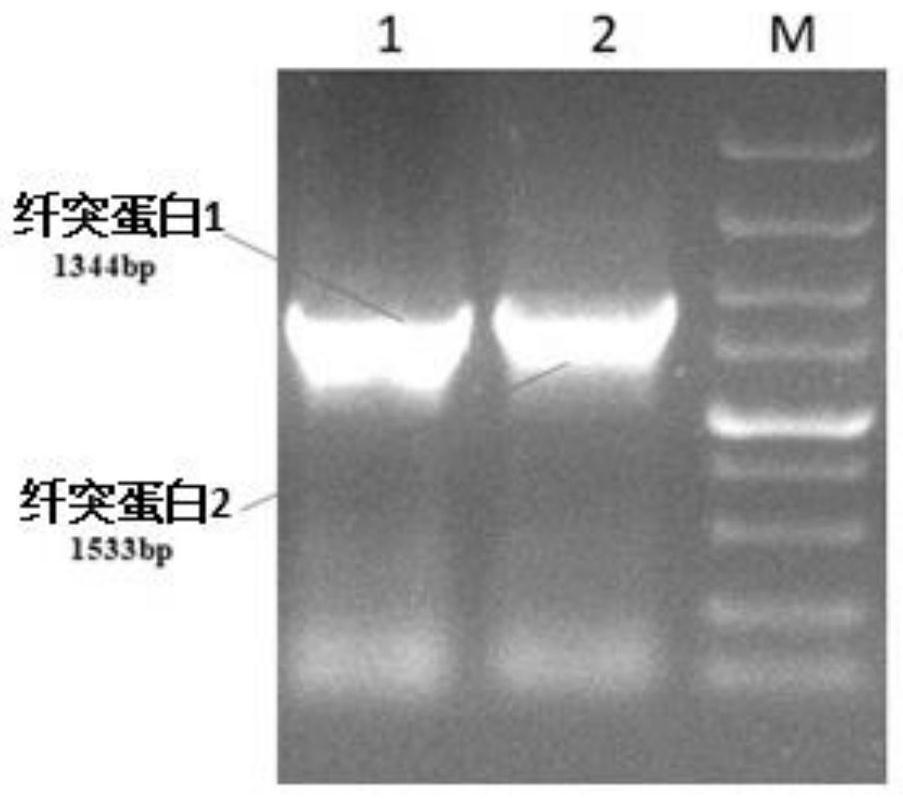
[0036] 2. PCR amplification of FAdV-4 spike protein 1 (fiber1) gene
[0037] (1) extract the DNA of FAdV-4 as template DNA, carry out the PCR amplification of fiber1 gene with specific primer P1 and P2;
[0038] (2) PCR amplification system: specific primer P1 1 μL, primer P2 1 μL, reaction solution contai...
Embodiment 2
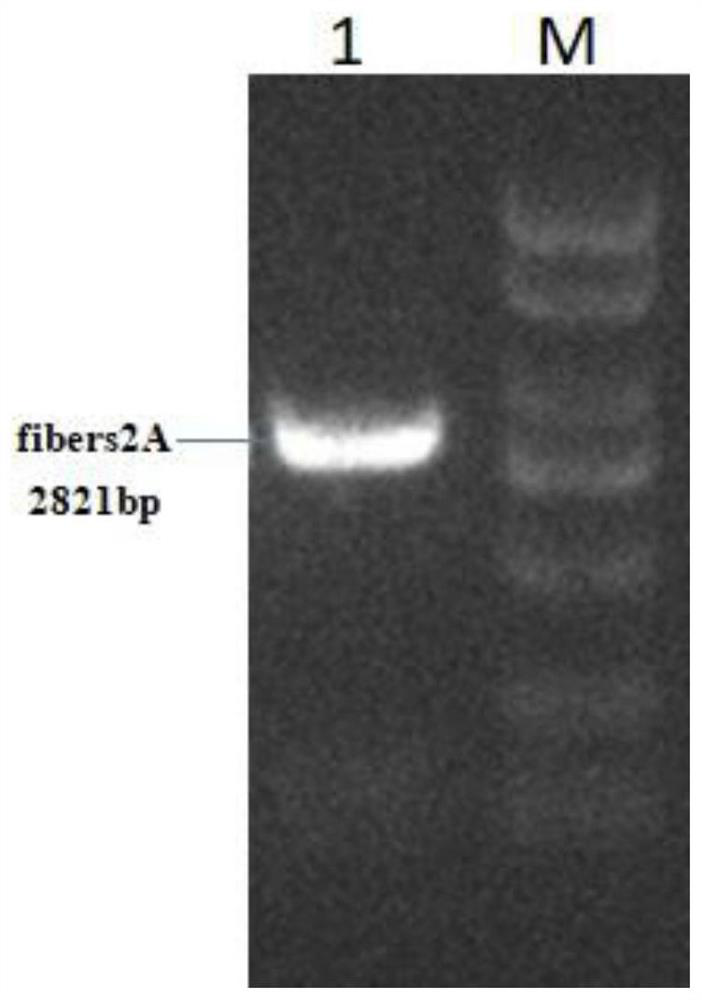
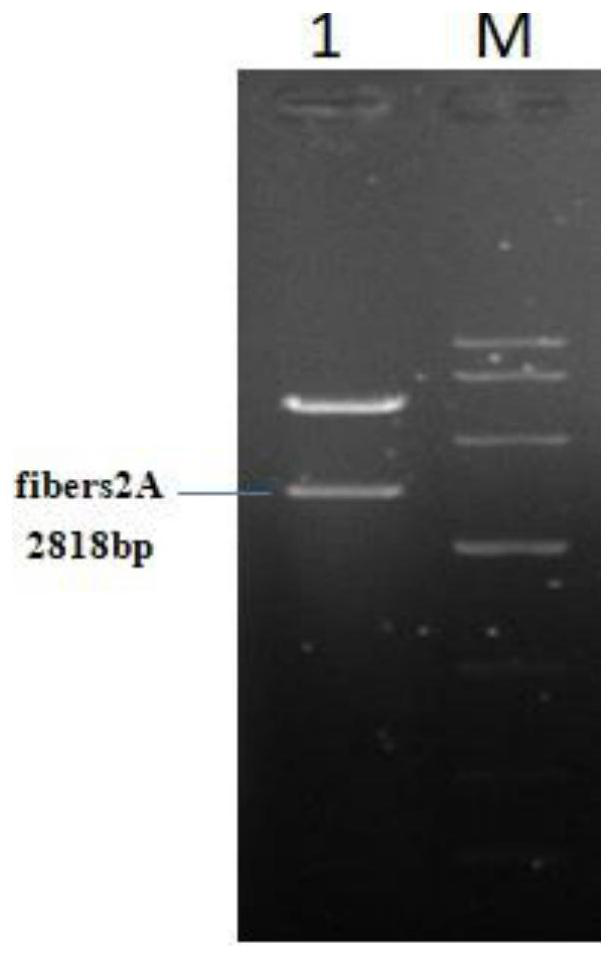
[0056] Example 2: Expression identification of recombinant plasmid pC-fibers2A
[0057] The recombinant plasmid pC-fibers2A constructed in Example 1 was transfected and grown into DF1 cells, and the expression of spike protein 1 and spike protein 2 were detected by immunofluorescence 48 hours after transfection, and the specific steps were as follows:
[0058] (1) Dissolve 600ng of the recombinant plasmid pC-fibers2A in 50μL DMEM, mix well and let stand at room temperature for 5min; at the same time, dissolve 2μL transfection reagent lipofectamine 2000 in 50μL DMEM, mix well and let stand at room temperature for 5min; The plasmid pC-fibers2A was mixed with the DMEM solution of transfection reagent lipofectamine 2000 to make the transfection solution. After standing at room temperature for 20 minutes, it was dropped into DF1 cells. After 8 hours of cultivation, it was replaced with DMEM containing serum. After 48 hours of continuous cultivation, it was used for immunofluorescenc...
Embodiment 3
[0060] Embodiment 3: DNA vaccine immunization test
[0061] (1) The recombinant plasmid pC-fibers2A was inoculated into 10 21-day-old SPF chickens, each inoculation amount was 200 μg, and the leg muscles were injected at 2 points, and booster immunization was carried out 14 days after the inoculation. The inoculation dose and method were the same as those of the first The second immunization was the same as the immunization group; at the same time, pCDNA3.1 empty vector injection was set as the control group, a total of 10 chickens;
[0062] (2) Challenge the virus 21 days after the booster immunization (the HN15 strain is FAdV-4, which is derived from the diseased chicken infected with FAdV-4), the challenge dose of each chicken is 100CLD50 (chicken half-lethal dose), and the virus challenge mode is Intramuscular injection, observe and record the morbidity and mortality of chickens;
[0063] (3) The results showed that some chickens in the control group began to fall ill on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com