Subunit vaccine, and preparation method and application thereof
A vaccine composition, Pasteurella porcine technology, applied in the direction of bacterial antigen components, antibacterial drugs, etc., can solve the problems of the immune effect of the attenuated live vaccine being susceptible to the environment, limited protective effect, and affecting virulence, so as to improve immune protection effects, avoiding adverse reactions, and reducing immunization costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1P.multocidaOmpA, OmpH, PlpE gene PCR amplification and construction of prokaryotic expression plasmid
[0058] 1.1 Design of primers
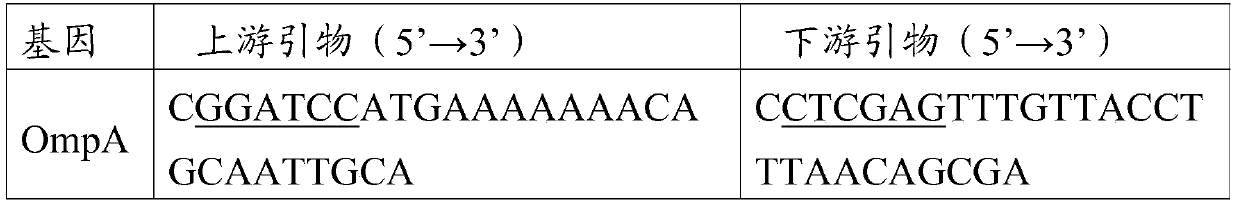
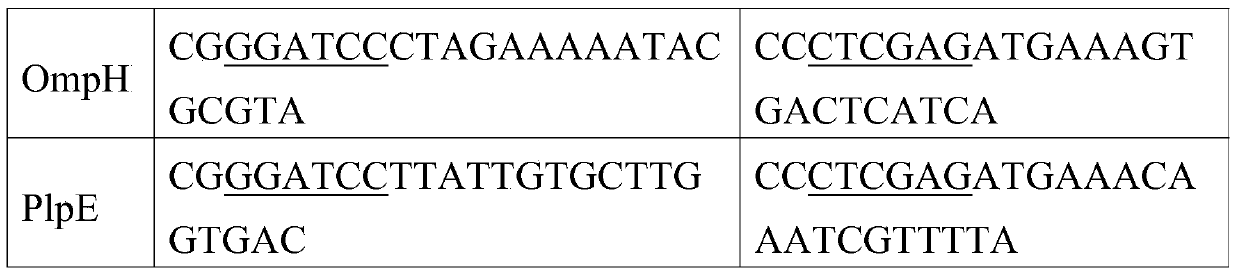
[0059] According to the OmpA gene sequence of the P.multocidapm-113 strain (accession number: JX435111.1) published in GenBank, the OmpH and PlpE gene sequences in the full-length gene sequence of the pm-70 strain (accession number: AE004439.1), use Primer5.0 software designed 3 pairs of primers respectively. The 5' ends of the upstream and downstream primers were respectively introduced with BamHI, XhoI restriction sites and protective bases to amplify the full-length sequences of OmpA, OmpH, and PlpE genes. The sequences of the 3 pairs of primers See Table 1, where the underlined part is the introduced restriction site, and the primers were synthesized by Invitrogen.
[0060] Table 1 Primer Sequence
[0061]
[0062]
[0063] 1.2 Extraction of Genomic DNA from P.multocida A Type HN5 Strain
[0064] Streak the Paste...
Embodiment 2
[0079] Example 2 Construction of recombinant expression strains BL21-OmpA, BL21-OmpH and BL21-PlpE
[0080] 2.1 Transformation of E.coliBL-21 Competent Cells with Recombinant Plasmid and Expression of Recombinant Protein
[0081] In Example 1, the recombinant plasmids sequenced correctly were transformed into E. coliBL-21 competent cells, and the recombinant expression strains BL21-OmpA, BL21-OmpH, and BL21-PlpE were respectively obtained. At the same time, the empty plasmid pET-32a was transformed into E. coliBL-21 in the same way. .coliBL-21 competent cells, coated with ampicillin-containing LB solid medium, cultured at 37°C for 16 hours, picked a single colony that grew well and inoculated them in 10ml of ampicillin-containing LB liquid medium, cultured with shaking at 37°C and 200rpm for 2h . OD of bacteria solution 600 When the value reaches 0.6, take 1 mL of uninduced expression bacteria solution and empty plasmid bacteria solution as samples before induction, add IPTG...
Embodiment 3
[0089] The preparation of embodiment 3Pm vaccine
[0090] 3.1 Preparation of recombinant Pm subunit vaccine
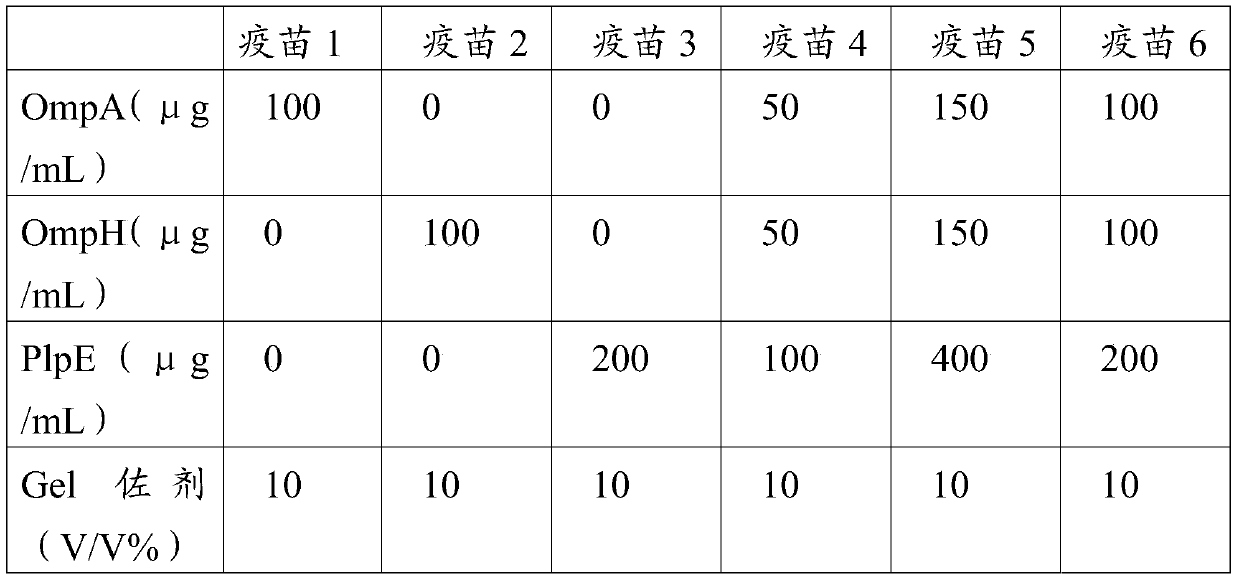
[0091] Combine r-OmpA, r-OmpH, and r-PlpE recombinant proteins according to a certain ratio, add a certain amount of Gel adjuvant (Seppic, France) and mix to make a Pm subunit vaccine containing three recombinant protein components with different concentrations , the concentrations of the three recombinant protein components in each vaccine are shown in Table 2.
[0092] Table 2 Protein content of different vaccines
[0093]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com