D-A spiro spiro skeleton configuration compound, synthetic method and application
A synthesis method and compound technology, applied in the field of fluorescent materials, can solve the problems of limiting the application of D-A type spirocyclic skeleton molecular frameworks, few synthesis methods, cumbersome reaction steps, etc., and achieve simple synthesis methods, simple reaction conditions and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This embodiment provides a kind of synthetic method of D-A spirocyclic skeleton structure compound, and this method comprises the following steps:
[0054] Step 1, in the glove box, add Pd(OAc) sequentially into the sealed tube 2(13.5mg, 0.06mmol), P(p-F-C 6 h 4 ) 3 (22.8mg, 0.072mmol), CsOPiv (42.1mg, 0.18mmol), Cs 2 CO 3 (560mg, 1.8mmol) and 60mL DMF were first stirred in the glove box for 5-10min, then compound 1 (320.6mg, 0.72mmol) and compound 2 (133.8mg, 0.6mmol) were added into the sealed tube. After the addition was complete, the sealed tube was taken out of the glove box and reacted at 130° C. for 20 h. After the reaction was completed, the reaction liquid was returned to room temperature, extracted with DCM and saturated ammonium chloride, and the extraction was repeated three times and the organic phase was collected. The organic phase was washed with anhydrous MgSO 4 Dry, filter with Celite, and spin dry. Separation by column chromatography (PE:EA=10...
Embodiment 2
[0075] This embodiment provides a kind of synthetic method of D-A spirocyclic skeleton structure compound, and this method comprises the following steps:
[0076] Step 1, in the glove box, add Pd(OAc) sequentially into the sealed tube 2 (135mg, 0.6mmol), P(p-F-C 6 h 4 ) 3 (228mg, 0.72mmol), CsOPiv (421mg, 1.8mmol), Cs 2 CO 3 (5.6g, 18mmol) and 60mL DMF were first stirred in the glove box for 5-10min, then compound 1 (3.2g, 7.2mmol) and compound 2 (1.3g, 6mmol) were added into a sealed tube. After the addition was complete, the sealed tube was taken out of the glove box and reacted at 130° C. for 20 h. After the reaction was completed, the reaction liquid was returned to room temperature, extracted with DCM and saturated ammonium chloride, and the extraction was repeated three times and the organic phase was collected. The organic phase was washed with anhydrous MgSO 4 Dry, filter with Celite, and spin dry. Separation by column chromatography (PE:EA=10:1, R f =0.30), t...
Embodiment 3
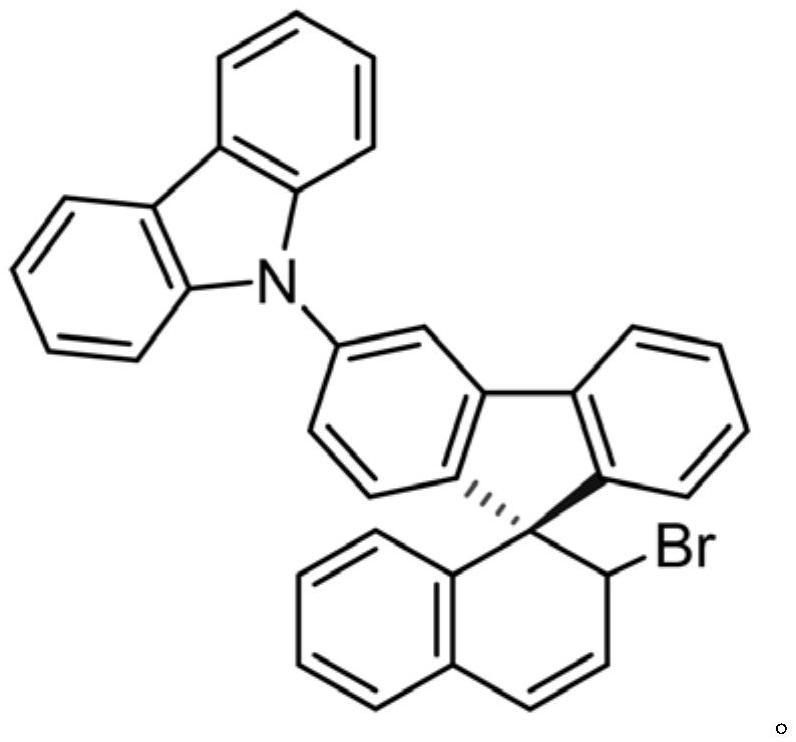
[0083] This example presents the application of the D-A spirocyclic skeleton compound as a fluorescent material. The D-A spirocyclic skeleton compound in this example uses the D-A spirocyclic skeleton compound obtained in Example 1.
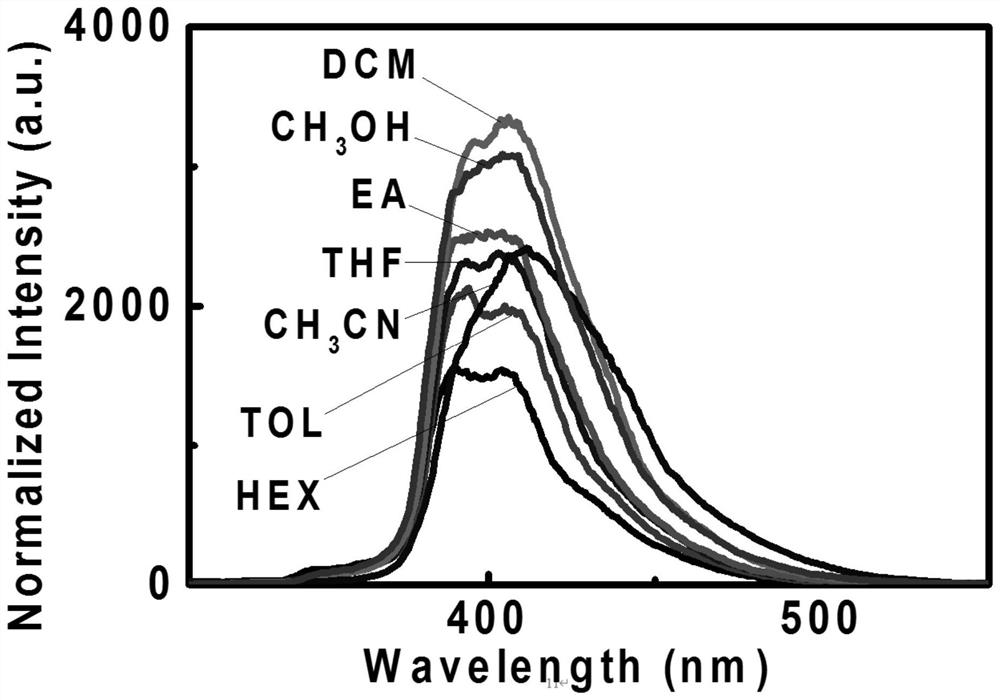
[0084] The D-A spiro ring skeleton type compound that obtains in embodiment 1 has carried out the mensuration of fluorescence emission spectrum, finds that it has better spectroscopic property, as figure 1 shown. It can be seen from its fluorescence emission spectrum that its peaks are all in the blue light region in different solutions. As the polarity of the solvent changes, the position of the emission peak does not change, but the emission intensity changes. Among them, the emission intensity is the largest in dichloromethane, and the emission intensity is the smallest in acetonitrile. It has broad application prospects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com