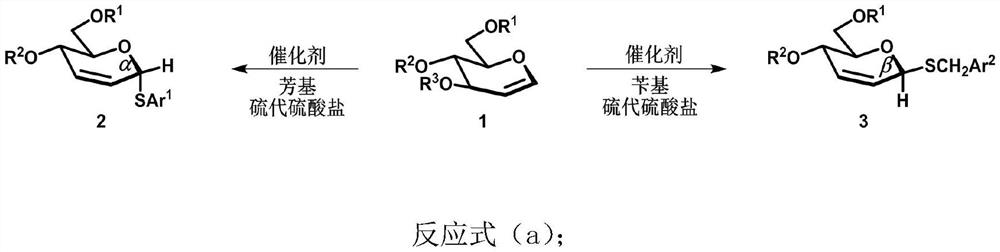
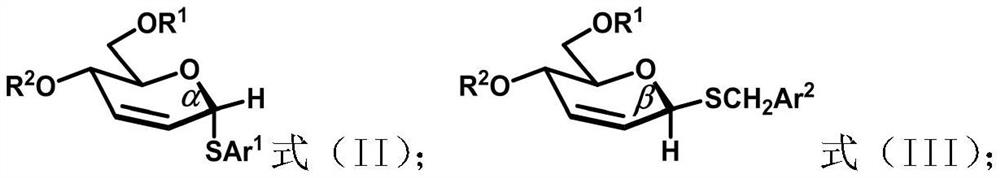
Unsaturated thioglycoside compound as well as selective synthesis method and application thereof
A synthetic method and compound technology, applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve the problems of limited substrate compatibility, poor selective synthesis, harsh reaction conditions, etc., and achieve universal substrate compatibility Wide, easy to handle, to achieve the effect of post-modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
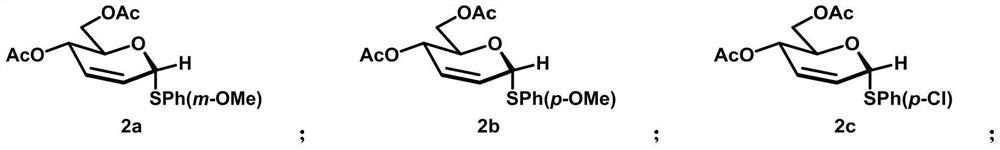
[0060] Synthesis of compound 2a:
[0061]
[0062] In a 25mL reaction tube, add triacetylglucosene (0.1mmol, 1.0equiv.), 3-methoxyphenyl thiosulfate (0.15mmol, 1.5equiv.), dibenzonitrile palladium dichloride (5.0mol %), (R)-(+)-1,1'-binaphthyl-2,2'-bisdiphenylphosphine (12.5mol%), acetonitrile (0.7mL, 0.075mmol / mL), evacuated for nitrogen, in The reaction was carried out at 90° C. for 12 hours. After the reaction was completed, it was dried, filtered, concentrated, and separated by column chromatography (PE / EA=5 / 1) to obtain a colorless liquid 2a (28.2 mg, 80%), Rf=0.5 ( PE / EA=5 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.14(d,J=8.2Hz,1H),7.09–7.04(m,2H),6.75(ddd,J=8.3,2.4,1.0Hz,1H),4.42–4.36(m,1H),4.18 (qd,J=12.1,4.3Hz,2H),3.76–3.71(m,3H),2.04(s,3H),2.00(s,3H). 13 C NMR (101MHz, CDCl 3 )δ170.9,170.3,159.8,136.0,129.7,128.5,127.7,123.7,117.2,113.2,83.6,77.3,77.03(s),76.7,67.3,65.1,63.0,55.3,21.0,20.8.IR(film,cm -1 ) 3055, 1649, 1180, 1130, 951, 896, 850, 746, 706. HRMS (EI) Calcd...
Embodiment 2
[0064] Synthesis of compound 2b:
[0065]
[0066] In a 25mL reaction tube, add triacetylglucosene (0.1mmol, 1.0equiv.), 4-methoxyphenyl thiosulfate (0.15mmol, 1.5equiv.), dibenzonitrile palladium dichloride (5.0mol %), (R)-(+)-1,1'-binaphthyl-2,2'-bisdiphenylphosphine (12.5mol%), acetonitrile (0.7mL, 0.075mmol / mL), evacuated for nitrogen, in The reaction was carried out at 90° C. for 12 hours. After the reaction was completed, it was dried, filtered, concentrated, and separated by column chromatography (PE / EA=5 / 1) to obtain a colorless liquid 2b (27.4 mg, 78%), Rf=0.5 ( PE / EA=5 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.14(d,J=8.2Hz,1H),7.09–7.04(m,2H),6.75(ddd,J=8.3,2.4,1.0Hz,1H),4.42–4.36(m,1H),4.18 (qd,J=12.1,4.3Hz,2H),3.76–3.71(m,3H),2.04(s,3H),2.00(s,3H). 13 C NMR (101MHz, CDCl 3 )δ170.9,170.3,159.8,136.0,129.7,128.5,127.7,123.7,117.2,113.2,83.6,77.3,77.03(s),76.7,67.3,65.1,63.0,55.3,21.0,20.8.IR(film,cm -1 ) 3055, 1649, 1180, 1130, 951, 896, 850, 746, 706. HRMS (EI) Calcd...
Embodiment 3
[0068] Synthesis of compound 2c:
[0069]
[0070] In a 25mL reaction tube, add triacetylglucosene (0.1mmol, 1.0equiv.), 4-chlorophenyl thiosulfate (0.15mmol, 1.5equiv.), dibenzonitrile palladium dichloride (5.0mol%) , (R)-(+)-1,1'-binaphthyl-2,2'-bisdiphenylphosphine (12.5mol%), acetonitrile (0.7mL, 0.075mmol / mL), evacuated for nitrogen, at 90°C Under the conditions of reaction for 12 hours, after the reaction was completed, it was dried, filtered, concentrated, and separated by column chromatography (PE / EA=5 / 1) to obtain a colorless liquid 2c (22.3mg, 58%), Rf=0.5 (PE / EA=5 / 1) EA=5 / 1). 1 H NMR (400MHz, CDCl 3 )δ7.41(d,J=8.4Hz,2H),7.26–7.11(m,3H),6.01–5.90(m,1H),5.81(d,J=10.1Hz,1H),5.64(s,1H ),5.30(dd,J=9.5,1.7Hz,1H),4.41–4.32(m,1H),4.27–4.07(m,2H),2.04(s,3H),2.00(d,J=3.8Hz, 3H). 13 C NMR (101MHz, CDCl 3 )δ170.7, 170.3, 133.9, 133.2, 129.1, 128.2, 128.0, 83.7, 67.4, 65.1, 63.1, 21.0, 20.8. IR (film, cm -1 ) 3053, 1653, 1180, 1130, 896, 854, 819, 746, 706. HRMS (EI) C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com