Multifunctional external ointment and preparation process thereof
A production process and multi-functional technology, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, antipyretics, etc., and can solve the problems of narrow use effect range, skin irritation, unfavorable use, etc. To achieve the effect of preventing and treating skin diseases, avoiding irritation and relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, the present embodiment provides a kind of technical scheme: a kind of multifunctional external ointment, described external ointment comprises the following components by weight: 5 parts of Sophora flavescens, 5 parts of duckweed, 5 parts of Kochia scoparia, 5 parts of Phellodendron Phellodendri 5 parts, white fresh skin 5 parts, 100 parts 5 parts, rhubarb 5 parts, cnidium 5 parts, wild chrysanthemum 5 parts, houttuynia cordata 5 parts, dandelion 5 parts, comfrey 5 parts, berberine 5 parts, indigo daisy 5 parts, 5 parts of green punt, 5 parts of Xu Changqing, 5 parts of cicada slough, 5 parts of silkworm, 2 parts of menthol, 2 parts of camphor, 2 parts of borneol, 5 parts of carbomer, 5 parts of sodium hyaluronate, 10 parts of yellow gelatin, and more The volume is deionized water.
[0017] A preparation process of a multifunctional external ointment, comprising the following steps:
[0018] S1. Material selection: First weigh Sophora flavescens, duckweed...
Embodiment 2
[0025] Embodiment 2, present embodiment provides a kind of technical scheme: a kind of multifunctional external ointment, described external ointment comprises the following components by weight: 10 parts of Sophora flavescens, 10 parts of duckweed, 10 parts of Kochia scoparia, 10 parts of Phellodendron Phellodendri 10 parts of white fresh skin, 10 parts of Baibu, 10 parts of rhubarb, 10 parts of cnidium, 10 parts of wild chrysanthemum, 10 parts of Houttuynia cordata, 10 parts of dandelion, 10 parts of comfrey, 10 parts of coptis, 10 parts of Qingdai, 10 parts of puny puffer, 10 parts of Xu Changqing, 10 parts of cicada slough, 10 parts of silkworm, 5 parts of menthol, 5 parts of camphor, 5 parts of borneol, 10 parts of carbomer, 10 parts of sodium hyaluronate, 15 parts of yellow gelatin, and more The volume is deionized water.
[0026] Wherein, the manufacturing process of the multifunctional external ointment in this embodiment is basically the same as that in Example 1, exc...
experiment example 1
[0028] Eye fatigue test: 60 volunteers were selected on a voluntary basis, aged between 30 and 40 years old, and all 60 volunteers selected had myopia between 400 and 500 degrees, and severe eye fatigue;
[0029] Divide 60 volunteers into four groups, A group and B group, 30 people in each group, 15 females and 15 males in each group;
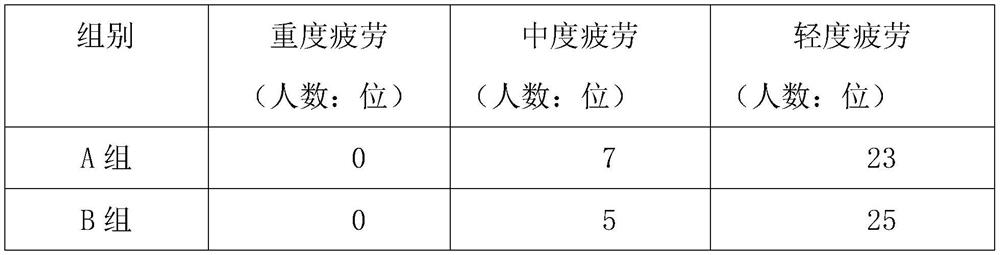
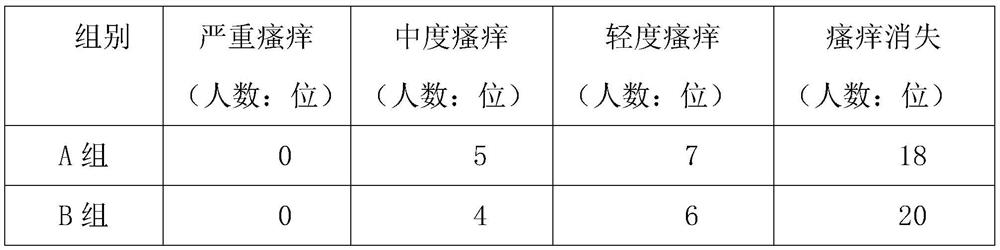
[0030] 30 volunteers in group A applied 2ml of the external ointment prepared in Example 1 to the corners of the eyes every day before going to bed, continued to use for 30 days, and checked the volunteers' eye fatigue after 30 days;
[0031] 30 volunteers in group B applied 2ml of the external ointment prepared in Example 2 to the corners of the eyes before going to bed every day, and continued to use it for 30 days. After 30 days, the volunteers were checked for eye fatigue;
[0032] After 30 days, under the same lighting environment, check the eye fatigue of each volunteer, and record the results in detail in Table 1 below. The order of eye ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com