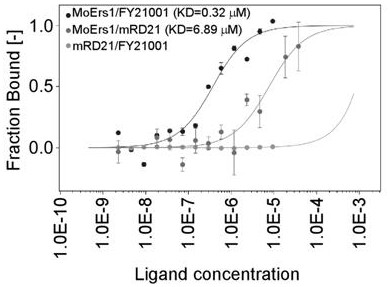
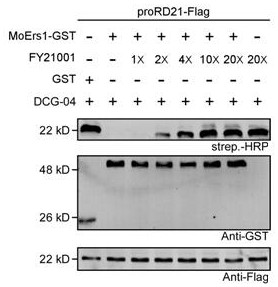
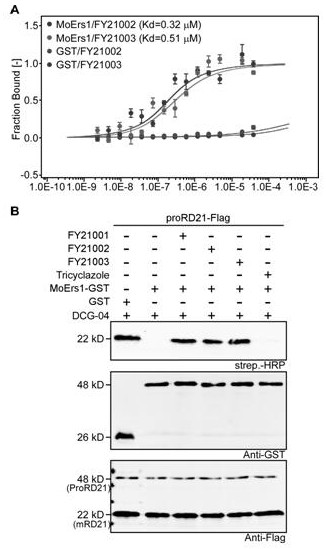
Diphenyl ether ester compound and application thereof, and pesticide bactericide
An ester compound and compound technology, applied in the field of organic chemical drugs, can solve problems such as drug resistance and environmental pollution, and achieve the effects of strong bactericidal activity, high safety and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the synthesis of compound II-1
[0070] The intermediate I used in this embodiment can be purchased directly, and the structure is as follows:
[0071]
[0072] Add intermediate I (5 mmol) to a 50 mL single-necked bottle, add 18 mL of dichloromethane, and slowly add (COCl) dropwise at room temperature 2 (8mmol), after the dropwise addition, the reaction was refluxed for about 1.5h, and the reaction progress was tracked by TLC, and the solvent was removed after the reaction. Add 20 mL of dry CH 2 Cl 2 , phenylpropanol (6 mmol), Et 3 N (10 mmol), reacted for about 0.5 h, followed by TLC until the acid chloride disappeared. After the reaction, wash once with 20 mL of water, wash twice with 10 mL of 1 mol / L HCl each time, wash with 10 mL of saturated NaHCO each time 3 Wash 2 times, recover the combined organic phase and wash with anhydrous Na 2 SO 4 After drying, petroleum ether ethyl acetate mixture was used as the eluent, separated and purified by ...
Embodiment 2
[0073] Embodiment 2: the synthesis of compound FY21001
[0074] The intermediate II-1 (5 mmol) prepared in Example 1, DMF (40 mL), potassium carbonate (10 mmol) and hydroquinone (10 mmol) were added into the flask, stirred and heated to 100°C. The reaction was monitored by TLC. After the reaction was completed, water (100ml) was added to the reaction system, ethyl acetate (30mL) was extracted three times, the organic layers were combined, and the organic phase was dried and concentrated for column chromatography to obtain compound FY21001, whose NMR spectrum was as follows :
[0075] 1 H NMR (500 MHz, DMSO- d 6 ) δ 9.47 (s, 1H), 7.90 – 7.84 (m, 2H), 7.27 –7.22 (m, 2H), 7.21 – 7.17 (m, 2H), 7.17 – 7.13 (m, 1H), 6.95 – 6.88 (m , 4H),6.81 – 6.76 (m, 2H), 4.18 (t,J = 6.5 Hz, 2H), 2.71 – 2.65 (m, 2H), 2.00 –1.91 (m, 2H).
Embodiment 3
[0076] Embodiment 3: the synthesis of compound FY21002
[0077] The intermediate II-1 (5 mmol) prepared in Example 1, DMF (40 mL), potassium carbonate (10 mmol) and o-cresol (10 mmol) were added into the flask, stirred and heated to 100°C. The reaction was monitored by TLC. After the reaction was completed, water (100ml) was added to the reaction system, ethyl acetate (30mL) was extracted three times, the organic layers were combined, and the organic phase was dried and concentrated for column chromatography to obtain compound FY21002.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com