Polypeptide for promoting skin repair and application thereof
A nucleic acid and selected technology, applied in skin repairing peptides and its application fields, can solve the problems of insufficient stability and dispersibility of cosmetics, achieve the effects of promoting collagen expression, anti-oxidation, good stability, and promoting skin repair Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The embodiment of the present invention also provides a preparation method of a polypeptide, which comprises: cultivating the host cell as described in the foregoing embodiments, or artificially synthesizing the polypeptide as described in any of the foregoing embodiments.
[0049] Conditions for culturing host cells can be obtained based on conventional techniques, as long as the host cells can express polypeptides, and will not be repeated here.
[0050] The embodiments of the present invention also provide the use of the polypeptide as described in any of the foregoing embodiments or the isolated nucleic acid as described in the foregoing embodiments in the preparation of a drug or cosmetic that promotes repair of damaged skin.
[0051] The embodiments of the present invention also provide the use of the polypeptide as described in any of the foregoing embodiments or the isolated nucleic acid as described in the foregoing embodiments in the preparation of drugs or cos...
Embodiment 1
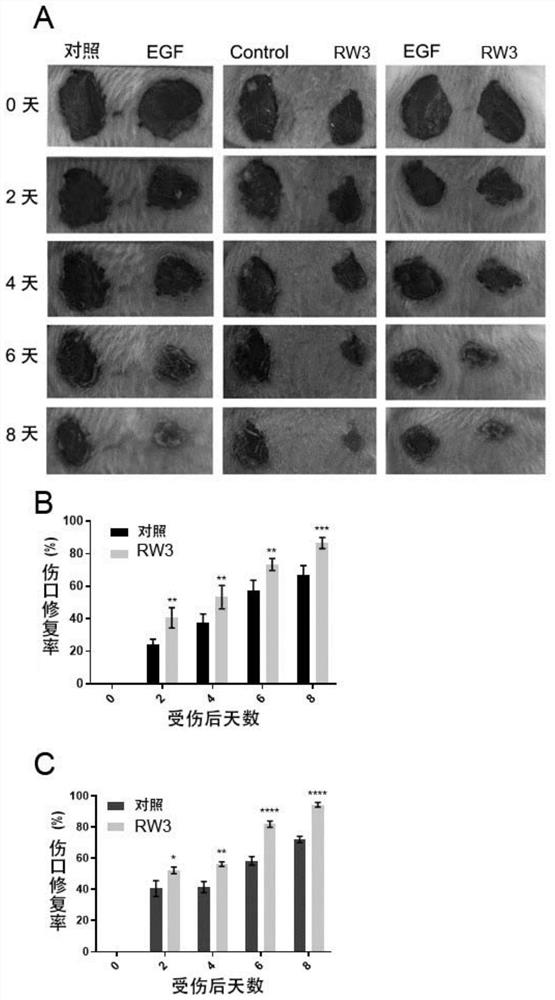
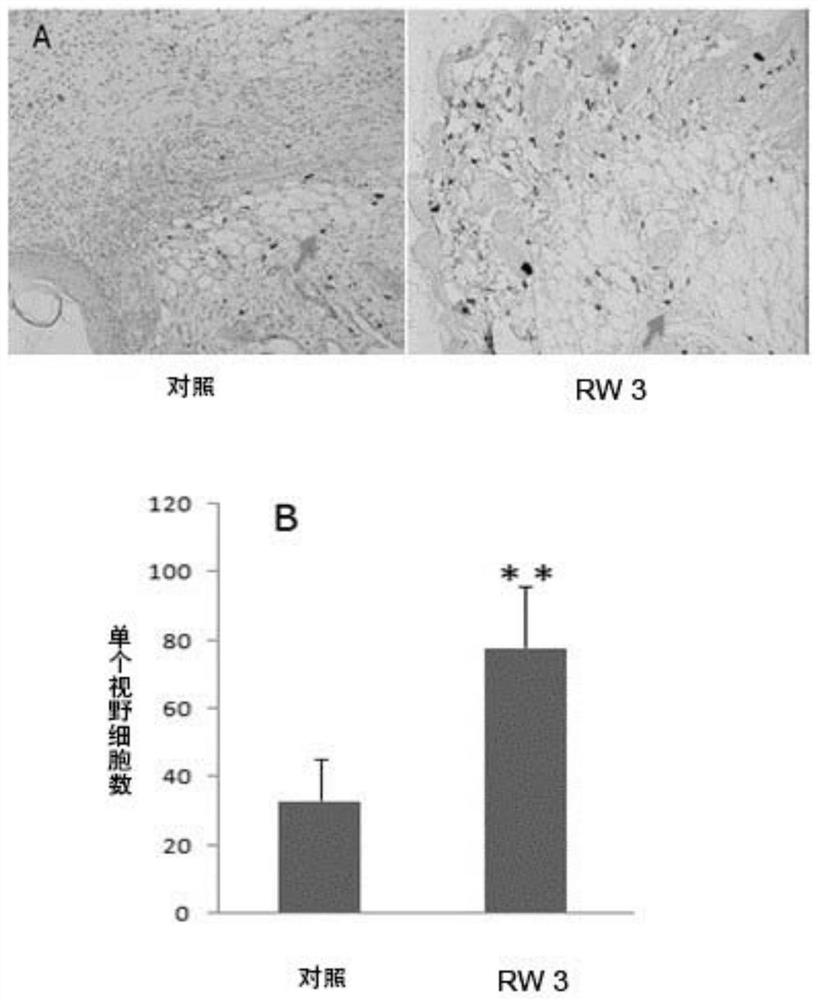
[0060] The present embodiment provides skin repair promoting polypeptide RW3, which is a linear polypeptide, and the primary structure of the complete amino acid sequence is: Met-Pro-Phe-Arg-Met-Gly-Ile-Cys-Thr-Met-Asn (MPFRMGICTMN ).
[0061] The preparation method of the skin-promoting peptide RW3, the specific steps are as follows:
[0062] 1. According to the designed amino acid sequence: Met-Pro-Phe-Arg-Met-Gly-Ile-Cys-Thr-Met-Asn, the crude polypeptide was synthesized by solid phase synthesis;
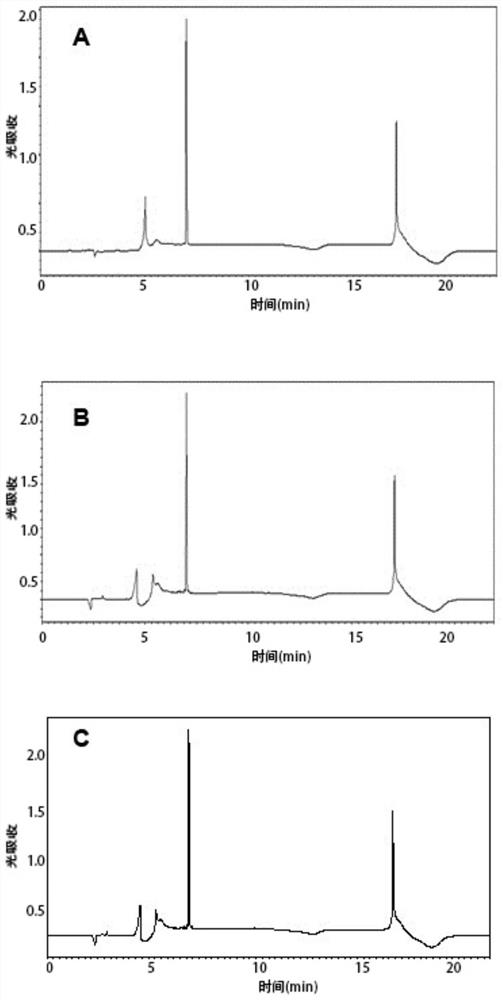
[0063] 2. Desalting and purifying the crude polypeptide by HPLC reverse-phase column chromatography, and identifying its purity until the purity of the polypeptide is not less than 95%;
[0064] HPLC purification and identification method: Dissolve 0.1mg of the sample to be tested in 1mL ultrapure water containing 0.1% trifluoroacetic acid. If there are undissolved impurities, filter them with a 0.45μm filter membrane. Mobile phase A is 0.1% trifluoroacetic acid - water, the mo...
Embodiment 2-8
[0076] Examples 2-8 respectively provide a polypeptide and its preparation method. The preparation method is roughly the same as that of Example 1, the difference is that the sequence is different. The amino acid sequence of the polypeptide provided by Examples 2-8 is as follows:
[0077] RW0: AMFRMGICTMN (SEQ ID No. 1);
[0078] RW1: AMFRMGMCTMN (SEQ ID No. 2);
[0079] RW2: APFRMGICTMN (SEQ ID No. 3);
[0080] RW4: MPFRMGMCTMN (SEQ ID No. 5);
[0081] RW5: MPFRMGMCTMM (SEQ ID No. 6);
[0082] RW6: PFRMGMC™ (SEQ ID No. 7);
[0083] RW7: PFRMGMCTMN (SEQ ID No. 8).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com