N,N-diphenylamino-modified beta-carboline indole onium salt as well as preparation method and application thereof
A technology of carboline indolium salt and diphenylamino, which is applied in chemical instruments and methods, preparations for in vivo experiments, pharmaceutical formulations, etc., and can solve the problem of inability to diagnose tumors in real time and dynamically observe tumors and normal tissues , Does not have fluorescence reversibility and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
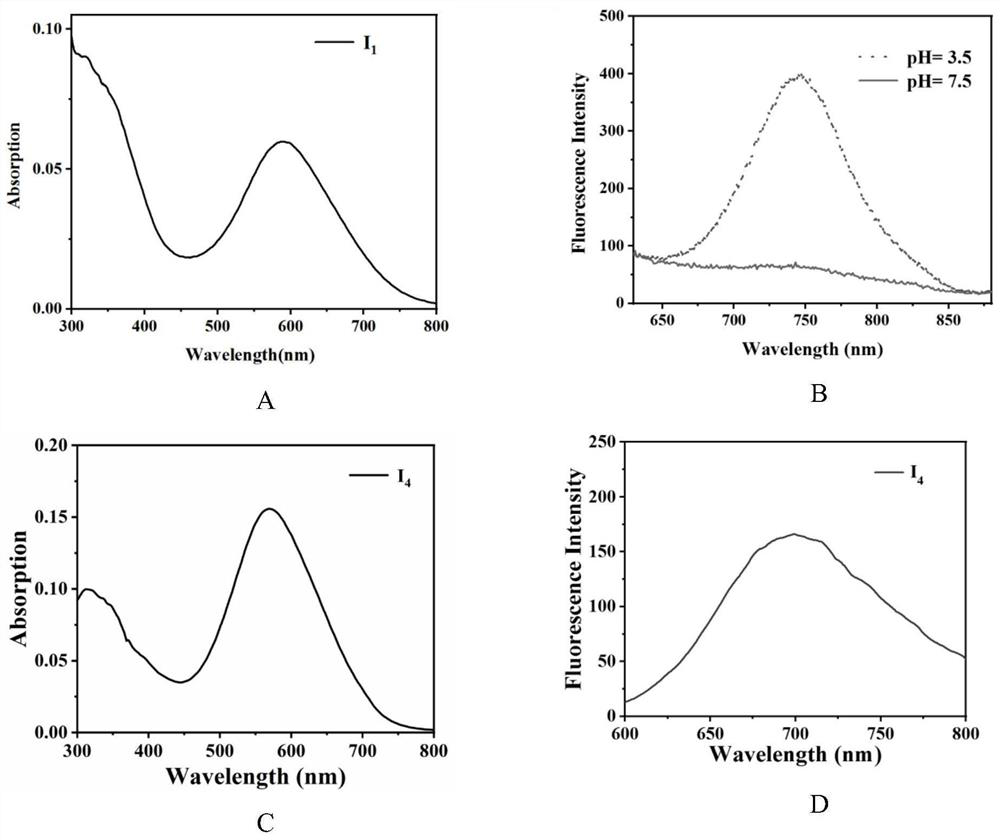
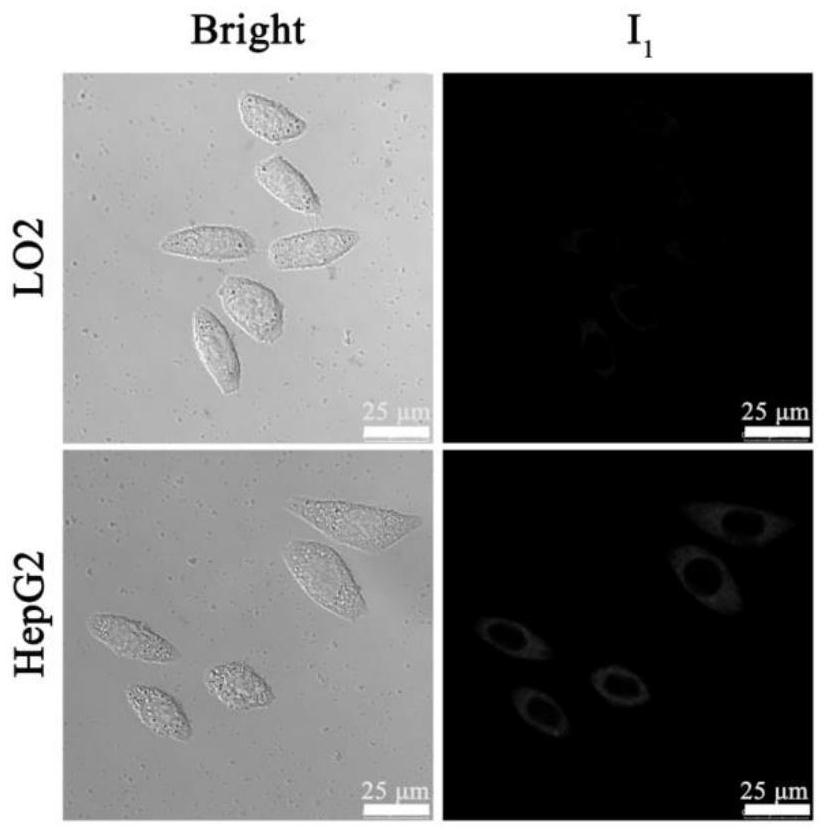
[0041] Example 1: (E)-2-(2-(6-(diphenylamino)-1,9-dimethyl-9H-pyrido[3,4-b]indol-3-yl)ethylene base)-7-iodo-1,1,3-trimethyl-1H-benzo[e]indole-3-iodide salt (compound I 1 )
[0042] (1) Preparation of 6-bromo-9-methyl-9H-pyridylmethyl[3,4-b]indole-3-carboxylate (compound 2)
[0043] Firstly, compound 1 (500mg, 1.57mmol) was dissolved in anhydrous DMF, and NaH (301mg, 12.56mmol) was added under ice-bath conditions and stirred for 0.5h, then CH 3 I (1ml, 15.7mmol), after continuing to stir at room temperature for 1h, after the reaction was monitored by TLC, the reaction solution was poured into ice water, adjusted to pH 7 with 0.1mol hydrochloric acid solution, the filtrate was suction filtered, and the filter cake was dried to obtain Compound 2, yield 85%. 1 H NMR (400MHz, DMSO) δ12.21(s,1H,NH),8.84(s,1H,ArH),8.66(d,J=1.7Hz,1H,ArH),7.70(dd,J=8.7,1.9 Hz,1H,ArH),7.61(d,J=8.7Hz,1H,ArH),3.90(s,3H,OCH 3 ),2.81(s,3H,CH 3 ).
[0044] (2) Preparation of 6-(diphenylamino)-1,9-dime...
Embodiment 2
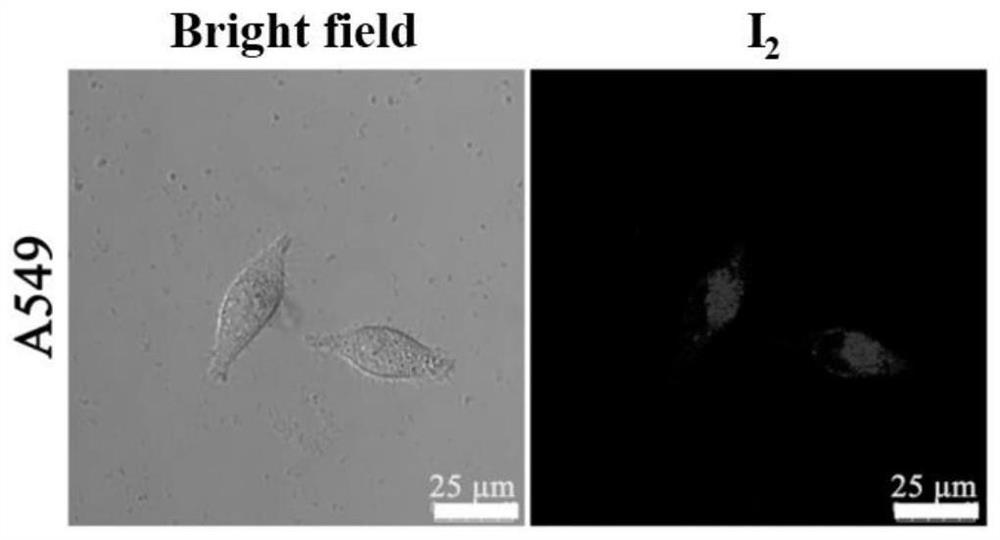
[0052] Example 2 (E)-2-(2-(6-(6-(diphenylamino)-1-methyl-9-(2-morpholinoethyl)-9H-pyrido[3,4- b]indol-3-yl)vinyl)-1,1,3-trimethyl-1H-benzo[e]indole-3-sulfonate (compound I 2 )
[0053] With reference to the preparation method of 2 in Example 1, the compound iodomethane is replaced by bromoethylmorpholine in the method, and finally the dark red product I is obtained. 2 , yield 76%. 1 H NMR(400MHz,DMSO)δ8.67(s,1H,ArH),8.23(d,J=2.1Hz,1H,ArH),8.23(m,2H,2ArH),8.06(m,2H,2ArH), 7.82 (d, J = 8.9Hz, 1H, ArH), 7.54 (m, 3H, 3ArH) 7.44 (d, J = 2.1Hz, 1H, ArH), 7.30 (m, 4H, 4ArH), 6.99 (t, J =8.2Hz, 6H, 6ArH), 4.78(dt, 2H, CH 2 ),3.54(m,4H,2CH 2 ),3.11(s,3H,CH 3 ),2.75(m,2H,CH 2 ),2.47(m,4H,2CH 2 ),2.06(s,6H,2CH 3 ),1.9(s,3H,CH 3 ).
Embodiment 3
[0054] Example 3 (E)-2-(2-(5-(6-(diphenylamino)-1,9-dimethyl-9H-pyrido[3,4-b]indol-3-yl) Thiophen-2-yl)vinyl)-1,3,3-trimethyl-3H-indole-1-bromide salt (compound I 3 )
[0055] (1) Preparation of 3-iodo-1-methyl-N,N-diphenyl-9H-pyrido[3,4-b]indol-6-amine (Compound 6)
[0056] First compound 3 (200mg, 0.508mmol), I 2 (280mg, 1.103mmol), K 3 PO 4 (186mg, 0.876mmol) was added into a sealed tube, dissolved in 2ml of acetonitrile, N 2 Protected, reacted at 110°C for 18h, after the reaction was completed, about 1.2ml of triethylamine was added to continue the reaction at 150°C for 4h, concentrated under reduced pressure, and separated by column chromatography to obtain compound 6 with a yield of 65%. 1 H NMR (400MHz, DMSO) δ8.70 (s, 1H, ArH), 8.19 (s, 1H, ArH), 7.79 (d, J = 8.9Hz, 1H, ArH), 7.42 (dd, J = 8.8, 1.9 Hz, 1H, ArH), 7.27(t, J=7.9Hz, 4H, 4ArH), 6.98(dd, J=10.7, 7.7Hz, 6H, 2CH=C, 4ArH), 4.21(s, 3H, CH 3 ),3.08(s,3H,CH 3 ).
[0057] (2) 5-(6-(diphenylamino)-1,9-dimet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com