Monoclonal antibody for resisting hepatitis B virus e antigen and application thereof
A technology of hepatitis B virus and monoclonal antibody, which is applied in the field of prevention and treatment, immunology, molecular virology, and diagnosis of hepatitis B virus, and can solve problems such as the limitation of HBeAg endpoint judgment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
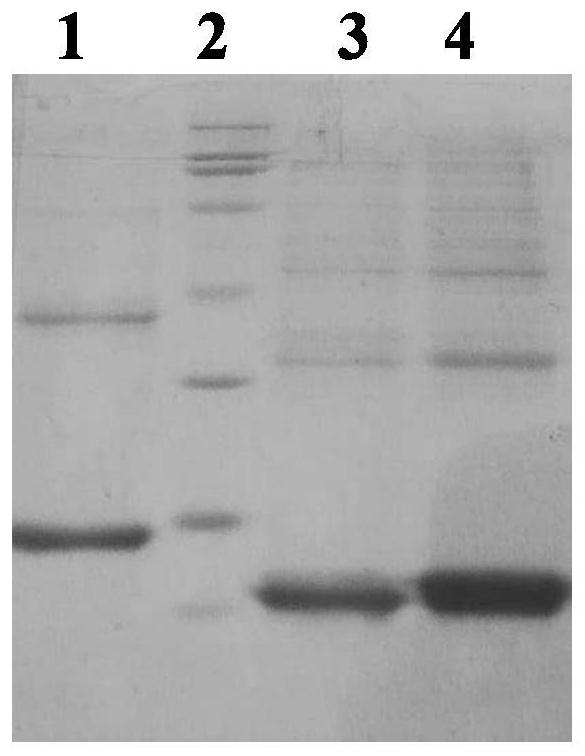
[0068] Example 1: Antigen Preparation
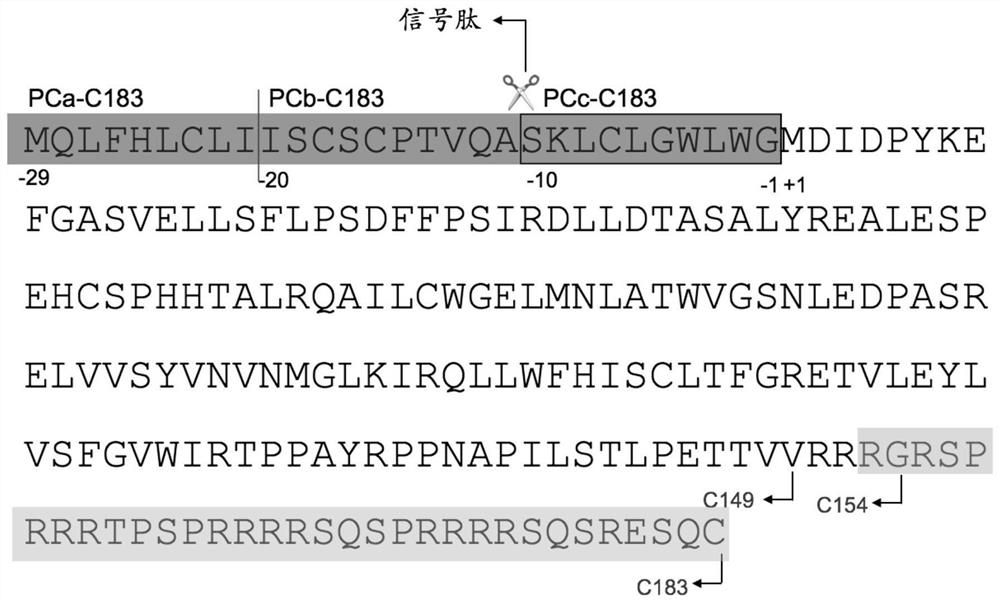
[0069] 1.1 Clone HBeAg -10-149, -10-152 and -10-183 amino acids
[0070] The -10-149 amino acid sequence of HBeAg is shown in SEQ ID NO:12, the -10-152 amino acid sequence is shown in SEQ ID NO:13, and the -10-183 amino acid sequence is shown in SEQ ID NO:14 Show. -10-149; -10-152; -10-183 antigens were prepared by using the Escherichia coli expression system.
[0071] 1.2 Preparation and purification -10-149; -10-152; -10-183 antigen
[0072] 1.2.1 Collect the bacterial liquid and ultrasonically crush it, centrifuge the crushed liquid at 12,000 rpm and 10°C for 10 min, and collect the supernatant.
[0073] 1.2.2 Precipitate the obtained supernatant with saturated thiamine, precipitate at 4 degrees for 1-2h, centrifuge at 13000rpm for 10min to collect the precipitate, and resuspend with 50mM TB8.8 buffer equal to the volume of the original supernatant, 1×PB7.4 Dialyze the sample. The sample was purified by medium-pressure DEAE-FF ...
Embodiment 2
[0074] Embodiment 2: Preparation of Anti-HBeAg mouse monoclonal antibody (hybridoma cell line 16D9, 9F10, 4C8, 14C12, 12D7, 8D1)
[0075] 2.1 Immunization of mice
[0076] 2.1.1 Preparation of the immunogen: the immunogen is -10-149, -10-152, -10-183 proteins expressed recombinantly in Escherichia coli. Take the completed antigen and dilute it to 0.4mg / mL, mix it with Freund's adjuvant in equal volume to form a water-in-oil emulsion (the method for judging whether the emulsification of the mixed solution is complete: a small drop of the mixed solution can be dropped on the liquid surface of clear water above, if the mixture does not condense, it can be considered to have been basically mixed). Complete Freund's adjuvant was used for the initial immunization, and incomplete Freund's adjuvant was used for subsequent booster immunization, and no adjuvant was added for the last booster immunization 72 hours before fusion.
[0077] 2.1.2 Basic immunization of mice: Use the ab...
Embodiment 3
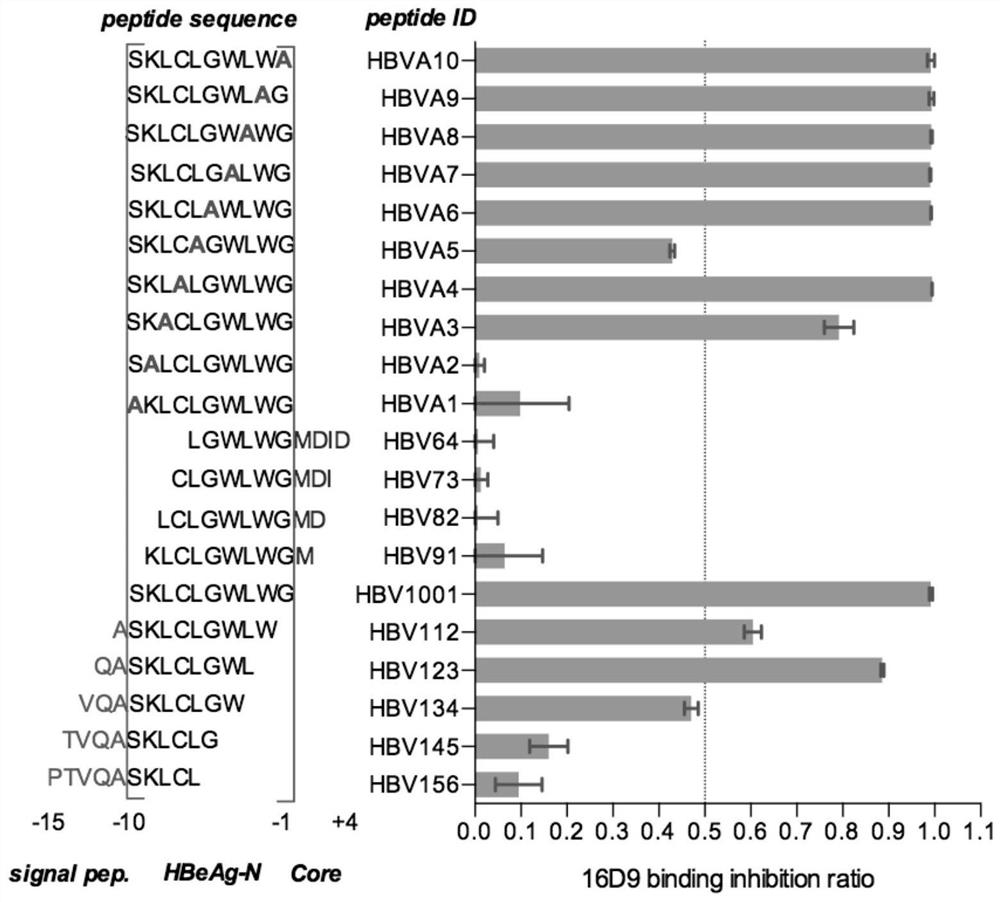
[0092] Example 3: Evaluation of the binding activity of Anti-HBeAg mouse monoclonal antibody to different synthetic peptides at the N-terminus of HBeAg
[0093] 3.1 Polypeptide synthesis
[0094] Using the HBV sequence (GenBank ID: AAL15969.1) as a reference sequence, 3 polypeptides were synthesized (synthesized by Shanghai Sangon Biotechnology Co., Ltd.). These three polypeptides (S1-S3) together cover the 10 amino acids from minus 10 to minus 1 of HBeAg. The peptide information of S1-S3 is shown in the table below.
[0095] Table 1: Peptide information of S1-S3
[0096]
[0097]
[0098] 3.2 Reactivity analysis of Anti-HBeAg mouse monoclonal antibody and peptide S1-S3
[0099] 3.2.1 Preparation of reaction plate
[0100] The peptide was treated with 50mM CB buffer (NaHCO 3 / Na 2 CO 3 buffer, the final concentration is 50mM, the pH value is 9.6), and the final concentration is 5μg / mL; add 100μL of coating solution to each well of a 96-well microplate plate, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com