Method for enhanced treatment of heavy metal complex wastewater
An enhanced treatment and complex technology, applied in chemical instruments and methods, special compound water treatment, water/sewage treatment, etc., can solve the problems of long hydraulic retention time, low removal efficiency, high treatment cost, etc., to ensure sufficient light , efficient treatment efficiency, and the effect of improving wastewater treatment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
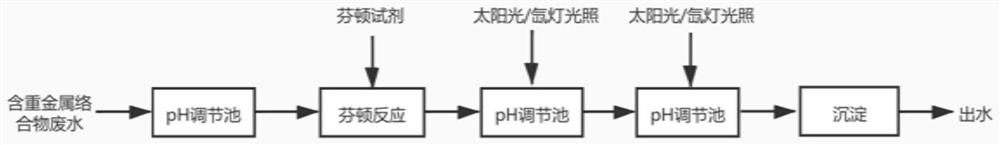
[0040] A kind of method for the intensive treatment of heavy metal complex waste water of the present embodiment, the waste water to be treated is the electroplating waste water containing carboxyl complexed nickel, wherein, the nickel concentration in the waste water is 18.4mg / L, COD is 127.5mg / L, And contain excess carboxyl. The specific processing steps are:
[0041]S10. Adjust the pH value of the waste water to 3, add 10mmol / L hydrogen peroxide, add 0.9mmol / L ferrous sulfate heptahydrate, and perform Fenton reaction for 5 minutes after mixing;
[0042] S20, adjust the pH value of the wastewater to 4, and irradiate it with a xenon lamp with a light intensity of 500W for 10 minutes;
[0043] S30, further adjusting the pH value of the wastewater to 8, and continuing to illuminate with a xenon lamp with a light intensity of 500W for 5 minutes;
[0044] S40, adjusting the pH value of the waste water to 12, stirring for 5 minutes, and standing for precipitation to obtain treat...
Embodiment 2
[0047] The basic content of this embodiment is the same as that of Example 1, except that the wastewater to be treated in this embodiment is wastewater containing copper complexes, wherein the copper concentration in the wastewater is 28.6 mg / L, and the COD is 150.0 mg / L. L, and contains excess tartaric acid. The specific processing steps are:
[0048] S10. Adjust the pH value of the waste water to 3, add 10mmol / L hydrogen peroxide, add 1.8mmol / L ferrous sulfate heptahydrate, and perform Fenton reaction for 5 minutes after mixing;
[0049] S20, adjust the pH value of the wastewater to 3.5, and irradiate it with a xenon lamp with a light intensity of 300W for 8 minutes;
[0050] S30, further adjusting the pH value of the wastewater to 8, and continuing to illuminate with a xenon lamp with a light intensity of 500W for 5 minutes;
[0051] S40, adjusting the pH value of the waste water to 9, stirring for 5 minutes, standing for precipitation, and obtaining treated waste water. ...
Embodiment 3
[0054] The basic content of this embodiment is the same as that of Example 1, except that the wastewater to be treated in this embodiment is wastewater containing copper complexes, wherein the copper concentration in the wastewater is 24.2 mg / L, and the COD is 200.3 mg / L. L, and contains excess citric acid. The specific processing steps are:
[0055] S10. Adjust the pH value of the waste water to 3, add 10mmol / L hydrogen peroxide, add 1.9mmol / L ferrous sulfate heptahydrate, and perform Fenton reaction for 5 minutes after mixing;
[0056] S20, adjust the pH value of the wastewater to 4, and irradiate it with a xenon lamp with a light intensity of 300W for 5 minutes;
[0057] S30, further adjusting the pH value of the wastewater to 8, and continuing to illuminate with a xenon lamp with a light intensity of 500W for 6 minutes;
[0058] S40, adjusting the pH value of the waste water to 10, stirring for 5 minutes, standing for precipitation, and obtaining treated waste water.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com