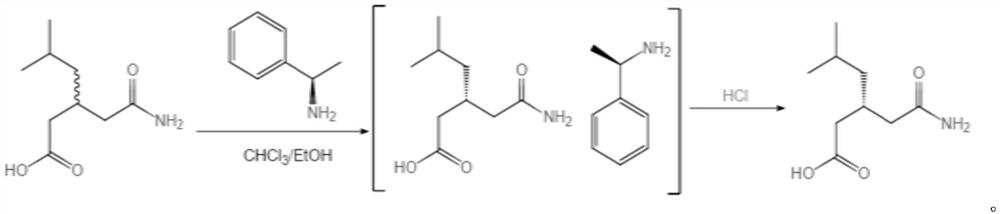
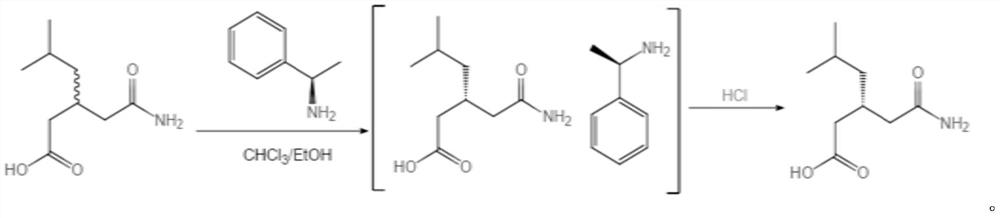
Refining method of high-purity R-(-)-3-carbamylmethyl-5-methylhexanoic acid
The technology of carbamoylmethyl and methylhexanoic acid is applied in the field of organic synthesis and refining, and can solve the problem of low purity of chiral monomer, R-(-)-3-carbamoylmethyl-5-methyl Insufficient purity of hexanoic acid, etc., to achieve the effect of high purity and good process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Put in 100g (±)-3-carbamoylmethyl-5-methylhexanoic acid, 2235g chloroform, 67g absolute ethanol, heat up to 55°C to dissolve, add 65g R-phenylethylamine, cool to 32± Filter at 1°C to obtain the wet product of R-(-)-3-carbamoylmethyl-5-methylhexanoic acid phenethylamine salt. Add the wet product of R-(-)-3-carbamoylmethyl-5-methylhexanoic acid phenethylamine salt and 187g of water into the reaction flask, heat up to 65°C to dissolve, let stand to separate layers, and the water layer is usually Pressure distillation to 72°C, then lower the temperature to below 40°C, add 47g of hydrochloric acid dropwise until the pH is 1-2, then cool to 25°C, heat and crystallize for more than 1 hour, filter with suction, rinse with water, and get R-(-)- 43 g of crude wet product of 3-carbamoylmethyl-5-methylhexanoic acid (38.2 g on dry basis, purity 94%, e.e. value 97.2%).
[0031] 2. Add 43g of the obtained R-(-)-3-carbamoylmethyl-5-methylhexanoic acid crude wet product, 90g of ethy...
Embodiment 2
[0033] 1. Put in 100g (±)-3-carbamoylmethyl-5-methylhexanoic acid, 2235g chloroform, 67g absolute ethanol, heat up to 55°C to dissolve, add 65g R-phenylethylamine, cool to 32± Filter at 1°C to obtain the wet product of R-(-)-3-carbamoylmethyl-5-methylhexanoic acid phenethylamine salt. Add the wet product of R-(-)-3-carbamoylmethyl-5-methylhexanoic acid phenethylamine salt and 187g of water into the reaction flask, heat up to 65°C to dissolve, let stand to separate layers, and the water layer is usually Pressure distillation to 72°C, then lower the temperature to below 40°C, add 47g of hydrochloric acid dropwise until the pH is 1-2, then cool to 25°C, heat and crystallize for more than 1 hour, filter with suction, rinse with water, and get R-(-)- 41 g of crude wet product of 3-carbamoylmethyl-5-methylhexanoic acid (37.4 g on dry basis, purity 95.2%, e.e. value 97.6%).
[0034] 2. Add 41g of the obtained R-(-)-3-carbamoylmethyl-5-methylhexanoic acid crude wet product, 90g of et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com