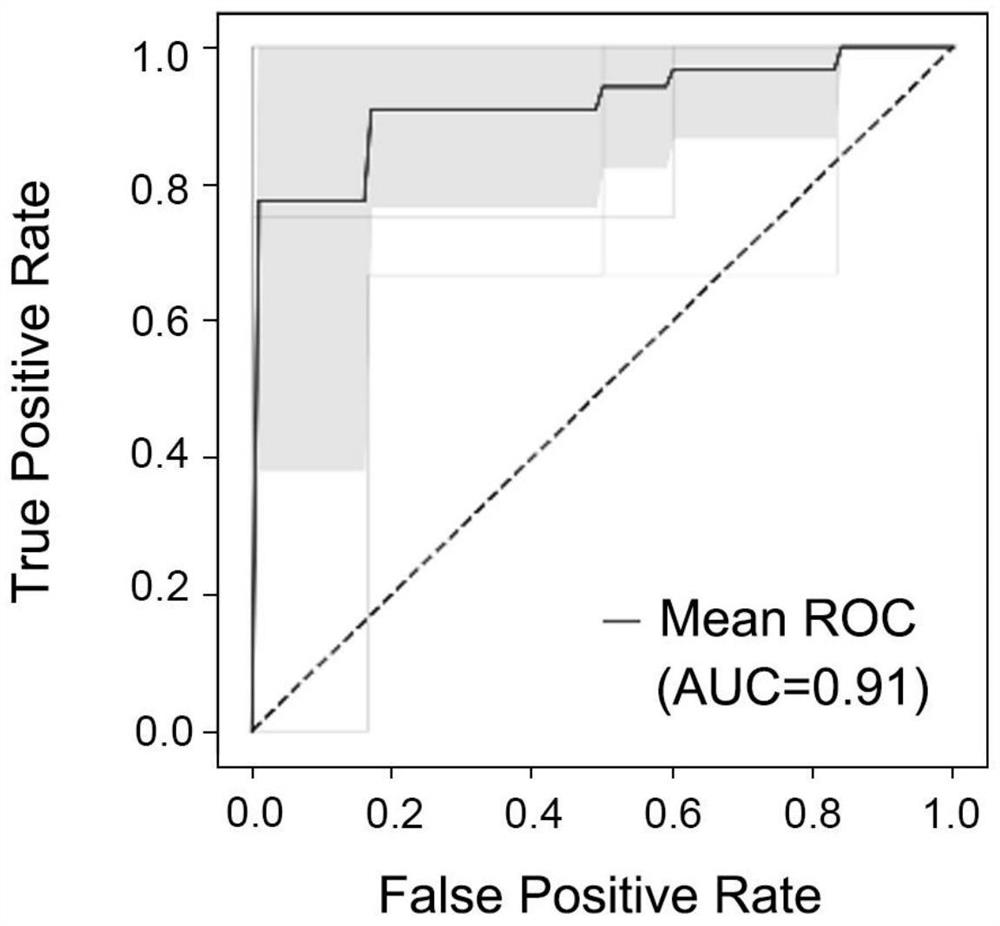
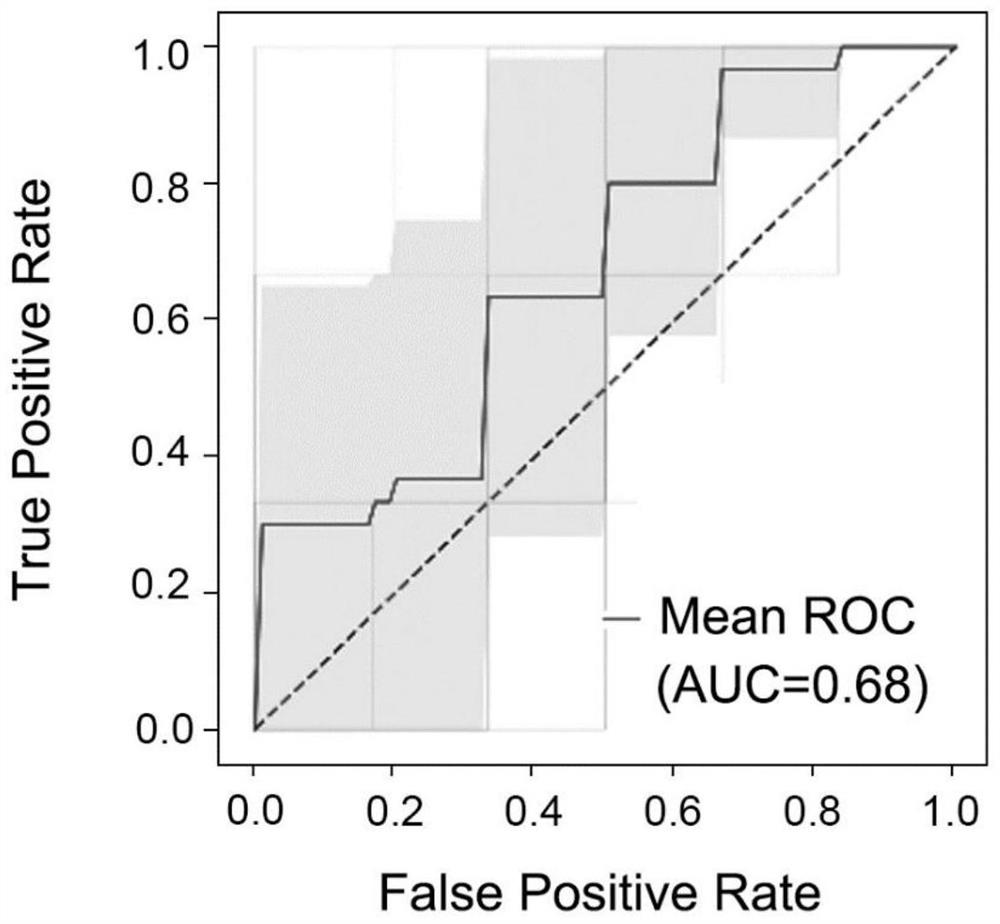
Application of peripheral blood exosome miRNA combined marker to preparation of kit for detecting HBV positive liver cirrhosis early liver cancer
A technology for detecting early and early stage liver cancer, applied in the fields of molecular biology and medical diagnosis, can solve the problems of lack of pertinence and complex pathogenesis of liver cancer, and achieve the effect of excellent sensitivity and specificity, convincing and accurate results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Subject and Sample Extraction
[0022] The samples were provided by Singapore General Hospital (approved by Singapore SingHealth CIRB B, approval number CIRB Ref: 2007 / 447 / B). Among them, 31 were LC+ / HBV+ HCC patients, and 59 were LC+ / HBV+ non-HCC patients.
Embodiment 2
[0023] Example 2 Extraction of exosomes
[0024] Collect 2ml of peripheral blood from the subject with a disposable syringe, quickly transfer it to an EDTA anticoagulant tube, and mix well. Separate the plasma within 2 hours of collection. The specific steps are: 1) transfer the whole blood to a 2ml centrifuge tube, and centrifuge at 500g for 10min at room temperature; 2) take the upper plasma, and centrifuge at 2000g for 10min at 4°C; 3) take the upper plasma, 4°C, Centrifuge at 16,000 g for 10 min; 4) The separated plasma is directly used for exosome extraction or frozen in a -80°C refrigerator.
[0025] Exosomes were extracted according to the instructions of the exosome extraction kit Exo-Quick exosome precipitation solution (EXOQ5SA-1, SBI, United States). 1) Add the plasma to a centrifuge tube, and add Exo-Quick at a ratio of 63 μl for every 250 μl of plasma; 2) Centrifuge at 1500 g at 4°C for 30 minutes, discard the supernatant; 3) Centrifuge at 1500 g at 4°C for 5 min...
Embodiment 3
[0026] Example 3 Extraction of exosome miRNA
[0027]miRNA was extracted according to the operating instructions of the miRNeasy Serum / PlasmaKit kit (217184, QIAGEN, Germany). The specific steps are: 1) add 5 times the volume of QIAzol Lysis Reagent to the plasma separated in Example 2, vortex or pipette to mix; 2) incubate at room temperature for 5 minutes; 3) add an equal volume of chloroform, and shake for 15 seconds; 4) Incubate at room temperature for 2-3 minutes; 5) Centrifuge at 12,000 g for 15 minutes at 4°C; 6) Transfer the upper layer to a new centrifuge tube, add 1.5 times the volume of absolute ethanol, and mix by pipetting; 7) Take 700 μl of the sample to the RNeasy MinElute spin column ( Place in a 2ml collection tube), cover the lid, centrifuge at room temperature>8000g for 15s, discard; 8) repeat step 7 once; 9) add 700μl RWTbuf to RNeasy MinElute spincolumn, centrifuge at room temperature>8000g for 15s, discard; 10) add Add 500μl RPEbuf to RNeasy MinElute spi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com