Application of apoptotic body in preparation of medicine for treating acute lung injury
A technology for acute lung injury and apoptotic bodies, applied in the direction of drug combination, drug delivery, and medical preparations containing active ingredients, etc., can solve the problems of damage to the patient's respiratory function, decline in the quality of life of the patient's prognosis, and incomplete clarity. To achieve the effect of improving the symptoms of acute lung injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 UC-MSCs apoptosis induction and AB extraction and identification
[0034] Culture UC-MSCs normally, add 10 μg / mL STS to induce for 12-16 hours after the confluence is greater than 80%, collect the supernatant of the culture medium, centrifuge at 800g for 10min, remove cell debris, take the supernatant, and centrifuge at 16,000g for 30min, the obtained precipitate is AB , resuspended in PBS, and frozen at -80°C for later use.
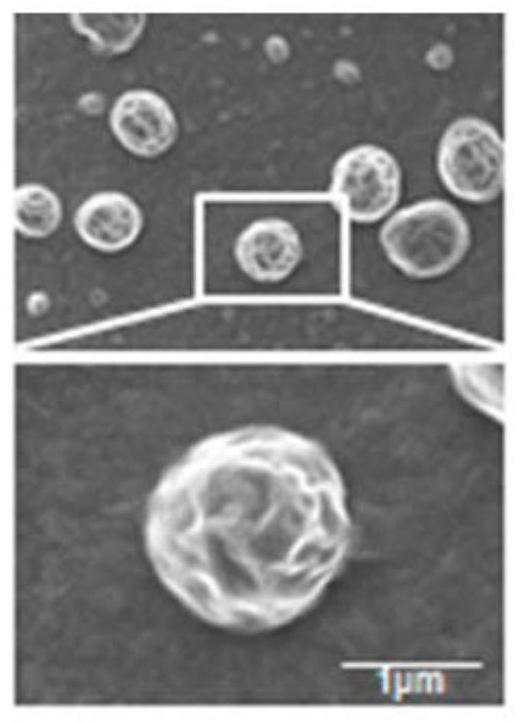
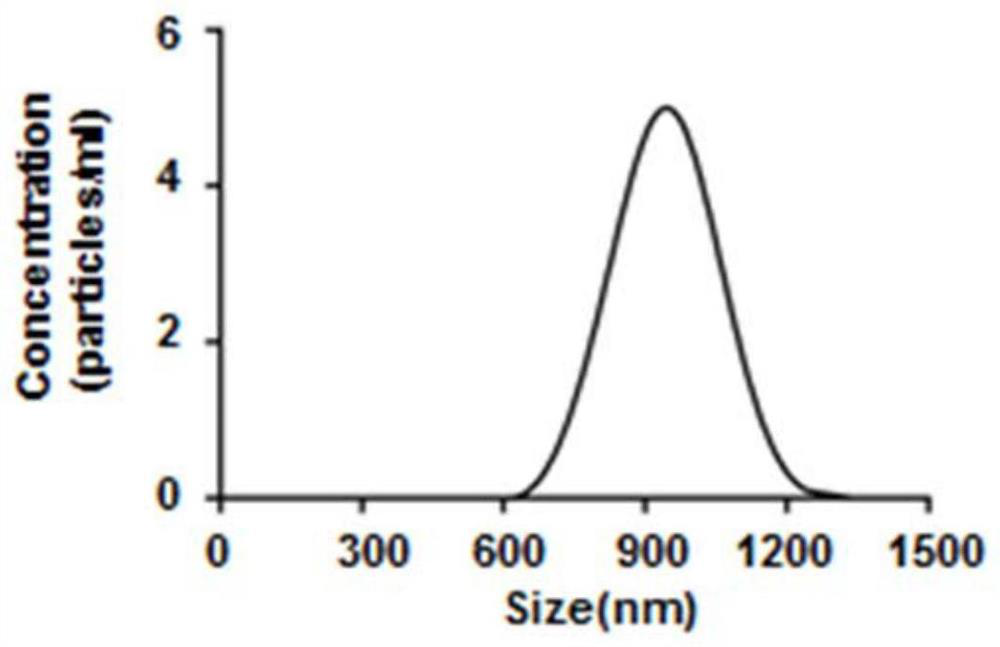
[0035] Take part AB and fix it with 2.5% (v / v) glutaraldehyde, drop it on the cover glass, dry, spray gold, observe with scanning electron microscope, as figure 1 The resulting AB shown has a complete membrane structure with a diameter of about 1 μm. The particle size analysis results show that the obtained AB particle size distribution is between 600nm and 1200nm, such as figure 2 shown.
[0036] Such as image 3 As shown, the resulting AB expressed Annexin-V. It was shown that the resulting AB expressed apoptotic body-specific su...
Embodiment 2
[0038] The establishment of embodiment 2 mouse acute lung injury model
[0039] Male C57 mice aged 7-8 weeks were selected and anesthetized as follows: Figure 5 As shown, the mouse model of acute lung injury was induced by instilling 20 μg LPS through the trachea with a 22GY intravenous indwelling needle oropharyngeally.
[0040] After the AIL model was established, the mice were randomly divided into three groups, PBS group, UC-MSCs treatment group and AB treatment group. At the same time, normal mice were used as controls. In the UC-MSCs treatment group, 1×10 6 Cells, mice in the AB treatment group were instilled with 10 μg / 20g AB through the trachea. After 24 hours, the therapeutic effect was detected.
Embodiment 3
[0041] Example 3 Detection of Therapeutic Effect of Acute Lung Injury in Mice
[0042] After treatment, the mice were taken, and the lung tissue was embedded in paraffin, sectioned, and stained with HE. It was found that both UC-MSCs and AB alleviated the damage to the lung tissue; Image 6 shown.
[0043] The alveolar lavage fluid was collected, and the expression of BALF protein and inflammatory factors IL-6 and TNF-α were detected by ELISA. The results showed that both UC-MSCs and AB could reduce the expression of inflammatory factors, but the effect of AB was more significant. Such as Figure 7 shown.
[0044] After the green fluorescent-labeled AB was instilled into the lung tissue of the mouse, the lung tissue was isolated, and after frozen section, the lung tissue was stained by immunofluorescence, in which DAPI (blue) marked the nucleus and F4 / 80 (red) marked the macrophage Cells, Ly6G (red) labeled neutrophils. Such as Figure 8 As shown, the infused AB (green fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com