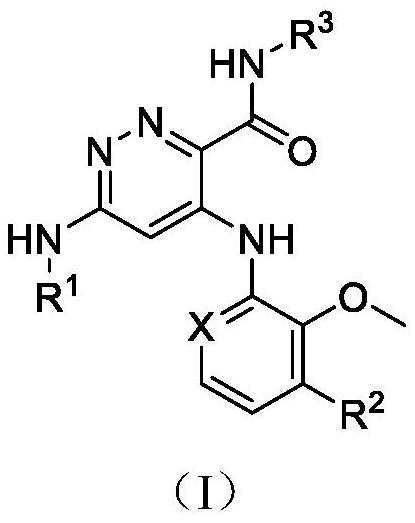
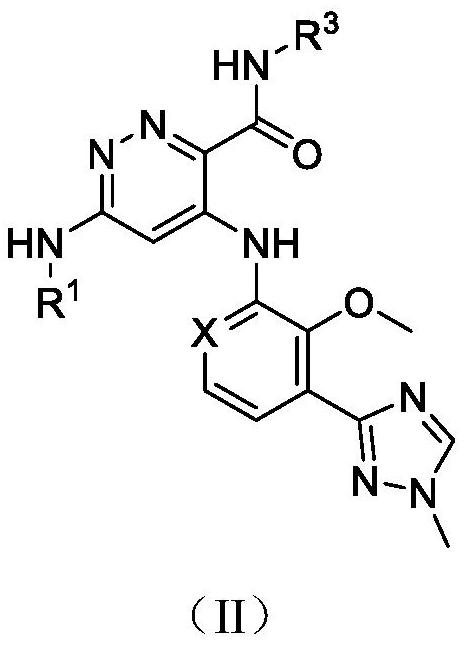
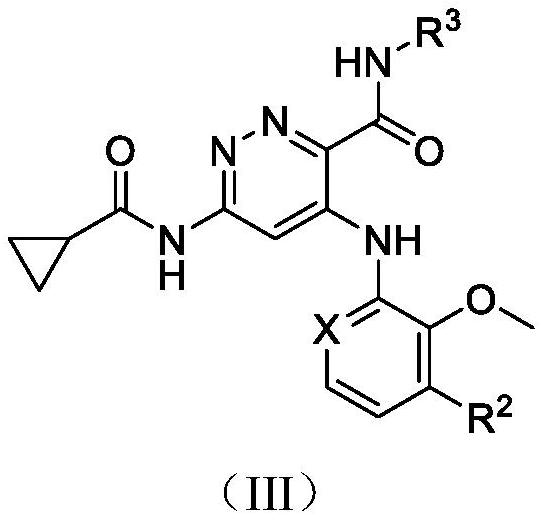
Pyridazine compound
A compound, pyridazine-based technology, applied in the field of TYK2 inhibitor, prevention or treatment of related diseases, can solve problems such as no observed toxicity performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Synthesis Example 1 001 Compound embodiment
[0096] 6- (cyclopropyl-carboxamido) -4 - ((2-methoxyimino-amine) ethyl) phenyl) amino) -N- methyl-pyridazine-3-carboxamide
[0097]
[0098] Synthetic route and specific synthetic steps:
[0099]
[0100] First step: 1- (2-methoxy-3-nitrophenyl) ethane-1-ketone 001-B synthesis
[0101] 001-A (3.0 g, 16.5 mmol) was dissolved in DMF (30.0 mL), and potassium carbonate (4.56 g, 33.0 mmol) was sequentially added, iodide (5.85 g, 41.25 mmol). The mixture was stirred for 12 hours, and the white solid was precipitated, filtered, collected, and ethyl acetate (30.0 mL) was added, and twice was washed with deionized water, and the brine was washed once. Thick, silica gel column chromatography was separated from 001-B (3.08 g, yield: 96%).
[0102] LCMS: RT: 1.797min; MS M / Z (ESI): 196.4 [M + H].
[0103] 1 H NMR (400MHz, CDCL 3 Δ7.93 (DD, J = 8.0 Hz, J = 1.6 Hz, 1H), 7.82 (DD, J = 8.0 Hz, J = 2.0 Hz, 1H), 7.30 (T, J = 8.0 Hz, 1H), 3.9...
Embodiment 2
[0124] Example 2 Synthesis of Compound 002
[0125] (E) -6 - ((5- (1- (hydroxyimine) ethyl) pyridin-2-yl) amino) -4 - ((2-methoxy-3- (1-methyl-1H- 1,2,4-triazole-3-yl) phenyl) amino) -N-methyl pyridazine-3-formamide
[0126]
[0127] Synthetic route and specific synthetic steps:
[0128]
[0129] Step 1: Starting materials 002-a synthesis
[0130] Starting materials and methods of Scheme 002-a of Ref. Highly Selective Inhibition ofTyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery ofthe Allosteric Inhibitor BMS-986165, J Med Chem.2019 Oct 24; 62 (20) : 8973-8995.
[0131] Step 2: 6 - ((5-acetylpyridine-2-yl) amino) -4 - ((2-methoxy-3- (1-methyl-1H-1,2,4-triazole-3 - Synthesis of Base) Phenyl) amino) -N-methylpyridazine-3-formamide 002-C
[0132] 002-A (65.0 mg, 0.48 mmol), 002-b (150.0 mg, 0.40 mmol), Pd at room temperature 2 (DBA) 3(37.0 mg, 0.04 mmol), xantphos (23.0 mg, 0.04 mmol) and cesium carbonate (261.0 mg, 0.80 mmol) were added to 1,4-dioxane...
Embodiment 3
[0138] Example 3 Synthesis of Compound 003
[0139] (E) -4 - ((2-methoxy-3- (1-methyl-1H-1,2,4-triazol-3-yl) phenyl) amino) -6 - ((5-) 1-methoxyamino) ethyl) pyridin-2-yl) amino) -N-methyl pyridazine-3-formamide
[0140]
[0141] Synthetic route and specific synthetic steps:
[0142]
[0143] 002-C (50 mg, 0.11 mmol), methoxy amine hydrochloride (18.5 mg, 0.22 mmol) and sodium acetate (36.0 mg, 0.44 mmol) were added to ethanol / water (3.0 ml / 6.0 mL). In the mixture, heated to 80 ° C for 18 hours, and the reaction solution was cooled to room temperature concentration. The crude product was dissolved in DMF (5.0 mL) by preparing liquid chromatography (0.1% ammonia water) purified from 003 (5.91 mg, yield 11%).
[0144] LCMS: RT: 4.60min; MS M / Z (ESI): 503.2 [M + H].
[0145] 1 H NMR (400 MHz, DMSO-D6) δ 11.01 (S, 1H), 10.36 (S, 1H), 9.16 (D, J = 4.8 Hz, 1H), 8.57 (S, 1H), 8.44 (D, J = 2.0Hz, 1H), 8.11 (S, 1H), 8.00 (DD, J 1 = 8.8Hz, J 2 = 2.0Hz, 1H), 7.70 (D, J = 8.8 Hz, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com