Genetically modified microorganism for producing 3-hydroxyhexanedioic acid, (E)-hex-2-enedioic acid and/or hexanedioic acid, and production method for the chemicals
A technology of adipic acid, hydroxyadipic acid, applied to genetically modified microorganisms for the production of 3-hydroxyadipic acid, alpha-hydrogenated adipic acid and/or adipic acid and the chemical In the field of manufacturing, it can solve problems such as no pyruvate kinase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
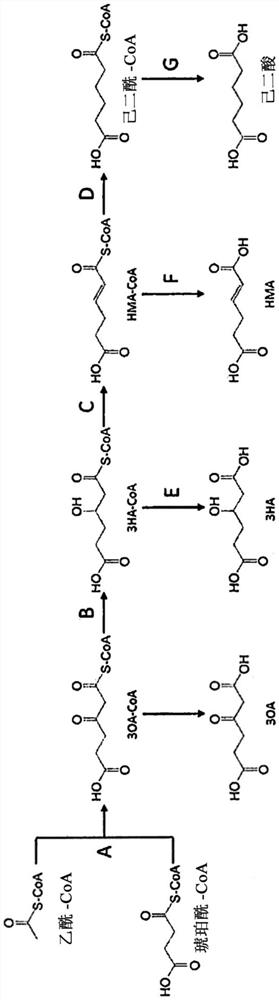
[0128] Preparation of 3-oxoadipyl-CoA solution: According to conventional methods, PCR was carried out with the genomic DNA of Pseudomonas putida KT2440 strain as a template, and the nucleic acids encoding CoA transferase (pcaI and pcaJ, NCBI-Gene ID: 1046613 and 1046612) were amplified in full length. In addition, the base sequences of the primers used for this PCR are SEQ ID NO: 194 and 195, for example. This amplified fragment was inserted into the KpnI site of pRSF-1b (manufactured by Novagen), which is an expression vector for Escherichia coli, so as to form the same frame as the histidine tag sequence. The plasmid was introduced into Escherichia coli BL 21 (DE3), and the expression of the enzyme was induced by using isopropyl-β-thiogalactopyranoside (IPTG) according to the conventional method, followed by the use of the histidine tag from the culture medium. Refined to obtain a CoA transferase solution. Using this solution, an enzyme reaction solution for producing 3-o...
Embodiment
[0240] Hereinafter, an Example is given and this invention is demonstrated concretely.
reference example 1
[0242] For the expression of enzymes that catalyze the reaction to form 3-oxoadipyl-CoA and coenzyme A (reaction A), the reaction to 3-hydroxyadipate from 3-hydroxyadipyl-CoA (reaction E ) and enzymes that catalyze the reaction (reaction F) to generate α-hydroadipiendioic acid from 2,3-dehydroadipyl-CoA, and Sequence numbers 1, 2, 3, 4, 5, 6, Production of the plasmid of the polypeptide described in 7
[0243] The self-replicating vector pBBR1MCS-2 in Escherichia coli (ME Kovach, (1995), Gene 166:175-176) was excised with XhoI to obtain pBBR1MCS-2 / XhoI. In order to assemble the constitutive expression promoter in this vector, the genomic DNA of Escherichia coli K-12MG1655 was used as a template to design the upstream region 200b (SEQ ID NO: 186) of gapA (NCBI Gene ID: NC000913.3). The primers (sequence numbers 187 and 188) for PCR amplification were subjected to PCR reaction according to conventional methods. The resulting fragment was ligated with pBBR1MCS-2 / XhoI using In-F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com