Composite adjuvant for animal vaccine, preparation method and vaccine
An adjuvant and vaccine technology, applied in the field of preparation of animal vaccines, can solve the problems affecting the absorption of adjuvants in the injection experience of finished products, and achieve the effects of stimulating humoral immune response, high antigen load, and avoiding viscosity increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Mix squalene, SPAN85 and TWEEN 80 in a weight ratio of 134:23:43, stir at a speed of 150rpm for 30min, and stand still to obtain a transparent solution;
[0043] (2) Prepare the water phase: the inactivated PCV2 cell culture that is qualified in the sterility test is the water phase;
[0044] (3) Mix the solution obtained in step (1) again, then add it to the water phase at a volume ratio of water phase: oil phase = 6:1, emulsify under stirring at 150 rpm, and stand to obtain an oil-in-water vaccine. Take a small amount of the finished product and drop it on the surface of cold water, and it spreads in the form of cloud mist, which is oil-in-water type; take 10ml of the finished product and centrifuge at 3000r / min for 15 minutes, no water phase precipitates at the bottom of the tube; store it at 4°C and 37°C for 28 days, no Separation occurs and the resulting vaccine is a stable emulsion.
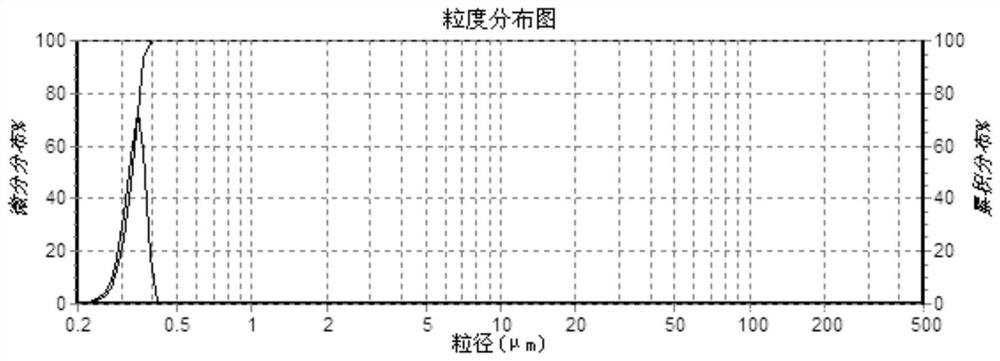
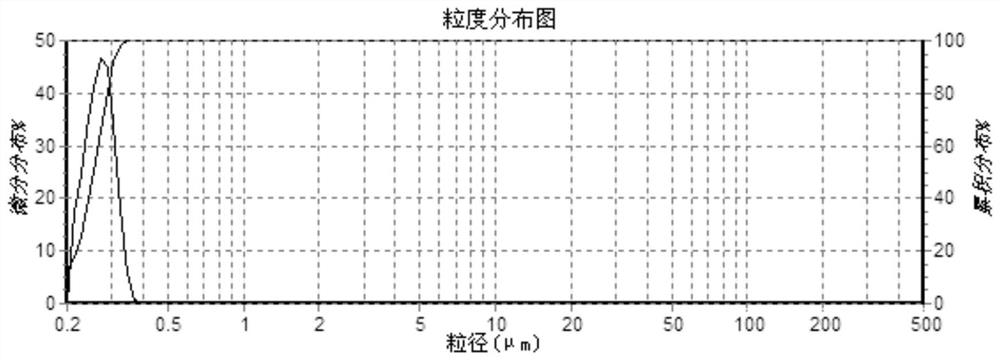
[0045](4) The finished product is measured with a rotary viscometer to have...
Embodiment 2
[0048] (1) Mix squalene, SPAN85, TWEEN 80 and saponin in a weight ratio of 12:2:5:1, stir at 150rpm for 40min, and stand still to obtain a transparent solution;
[0049] (2) Prepare the water phase: the inactivated PCV2 cell culture that is qualified in the sterility test is the water phase;
[0050] (3) Re-mix the solution obtained in step (1), then add it to the water phase at a volume ratio of water phase: oil phase = 8:1, emulsify under stirring at 150 rpm, and stand to obtain an oil-in-water vaccine. Take a small amount of finished product and drop it on the surface of cold water, and it spreads in the form of cloud, which is oil-in-water type; take 10ml of finished product and centrifuge at 3000r / min for 15 minutes, no water phase precipitates at the bottom of the tube; store at 4°C and 37°C for 28 days, no Stratification occurs and the resulting vaccine is a stable emulsion.
[0051] (4) The finished product is measured with a rotary viscometer to have a viscosity of 5...
Embodiment 3
[0061] Embodiment 3: mouse immune test
[0062] 1. Test materials
[0063] Table 1: Vaccine Information
[0064]
[0065] Table 2: Kit information
[0066]
[0067] 3. Experimental mice: purchased from Hangzhou Normal University, about 30 days old. Among them, there were 11 male mice and 14 female mice. Every 5 male mice were divided into 1 group, a total of 2 groups, and the remaining 1 was used as the control group; every 5 female mice were divided into 1 group, a total of 2 groups, and the remaining 4 were used as the control group.
[0068] 2. Immunization procedure: one group (5 rats / group) of male mice and one group of female rats were selected to be immunized with 0.2ml / mouse of the cyclinox vaccine; the remaining two groups (5 rats / group) were immunized with HS1010 emulsified vaccine, 0.2ml / mouse; The control group was injected with normal saline, 0.2ml / rat.
[0069] Twenty-eight days after the first immunization, 2 animals were selected from the Verdil vacc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com