Bacterial toxin vaccine and application thereof in preventing bacterial infection
A technology of bacterial toxins and vaccines, which is applied in the field of related and genus health, and can solve problems such as the difficulty in maintaining the activity of red blood cell membranes, the complicated process of coating nanoparticles on cell membranes, and the difficulty in large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the construction of liposome vaccine
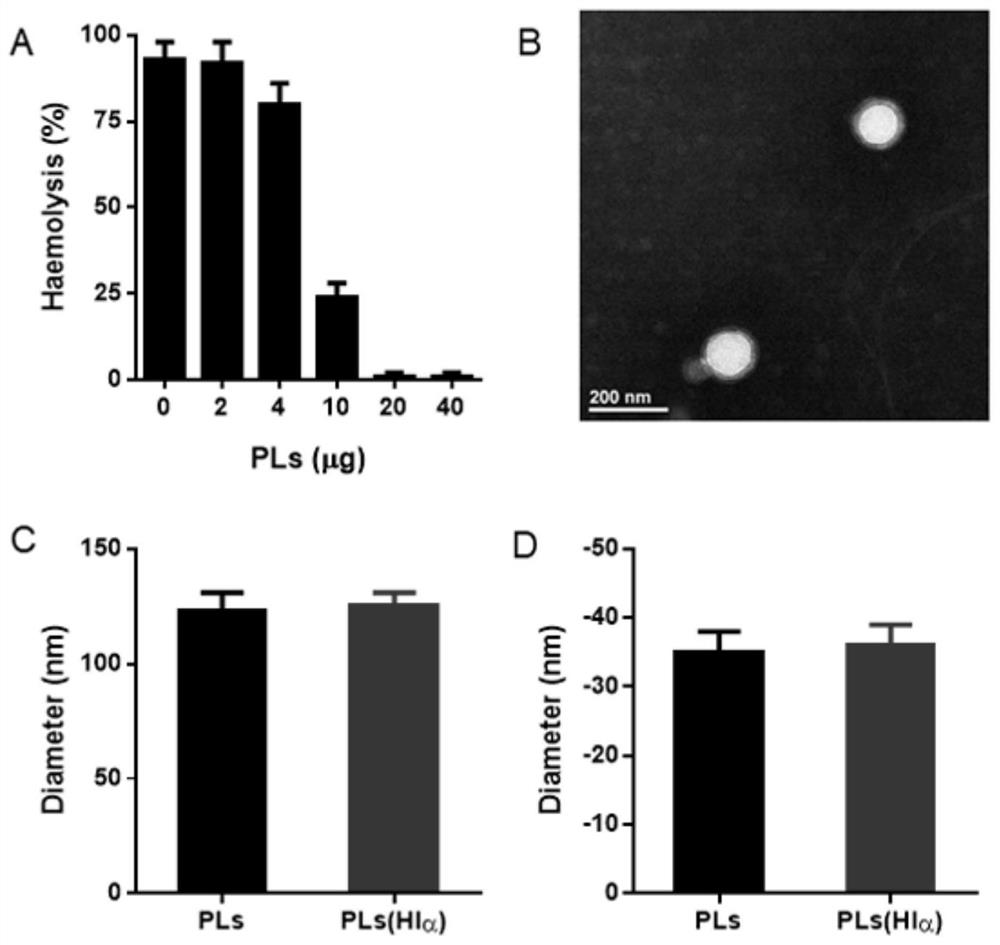
[0037] In this example, high cholesterol liposomes were first prepared by film hydration extrusion method. Specific operation: Weigh cholesterol, phosphatidylcholine (PC), DSPE-PEG2000 and / or sphingomyelin (as shown in Table 1) and dissolve them in dichloromethane, and spin evaporate at room temperature to form a lipid film. Add an appropriate amount of deionized water and stir at room temperature for 1 hour of hydration, 100W ultrasonic to obtain liposome (PLs) suspension. The toxin load of PLs was determined by in vitro hemolysis assay. Specific operation: Incubate 3 μg Hlα with different amounts of PLs at room temperature for 30 minutes, then incubate with appropriate amount of 2.5% RBC at 37°C for 3 hours, centrifuge at 2000 g for 5 minutes, measure the absorbance value of the supernatant at 540 nm and calculate the percentage of hemolysis according to the following formula. The negative control is physiological...
Embodiment 2
[0041] Embodiment 2: Physicochemical property characterization of liposome vaccine
[0042] The high-cholesterol liposomes (PLs) without toxin were used as the control. After negative staining with uranyl acetate, the shape and size of PLs(Hlα) were observed by transmission electron microscopy, and the results showed ( figure 1 B), Fused liposomes are regular spherical and uniform in size, similar to PLs. The average particle size, particle size distribution and polydispersity coefficient of PLs (Hlα) were measured by dynamic light scattering method, and its Zeta potential was measured. The results showed that the average particle size of PLs (Hlα) was 125nm, and the particle size distribution was narrow, and the potential was -35mV, which was similar to PLs ( figure 1 C, D). The above results indicated that the prescription amount of toxin loading had no significant effect on the physicochemical properties of fusion liposomes.
Embodiment 3
[0043] Example 3: Safety Evaluation of Liposome Toxoid Vaccine
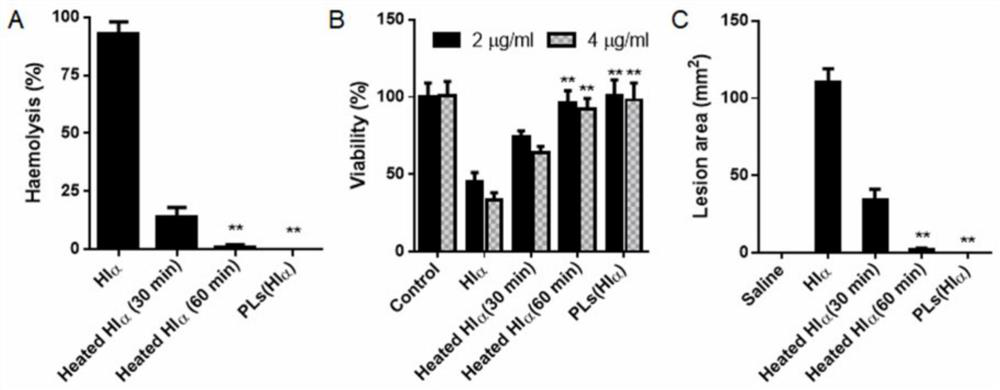
[0044] In this example, the in vitro safety of PLs (H1α) was first investigated through hemolysis experiments, and compared with H1α and heat-inactivated H1α. Specific operation: Hlα, heat-inactivated Hlα (70°C for 30min or 60min), PLs, PLs(Hlα) were shaken and incubated with 2.5% RBC for 3 hours at 37°C, then centrifuged at 2000g for 5min, and the absorbance of the supernatant at 540nm was measured Value and calculate the percentage of hemolysis according to the formula of Example 1. the result shows( figure 2 A), Hlα inactivation at 70°C for 30 minutes still has a certain hemolysis effect, and inactivation at 70°C for 60 minutes can completely eliminate the hemolysis effect of Hlα, while PLs (Hlα) has no hemolysis effect, showing better safety.
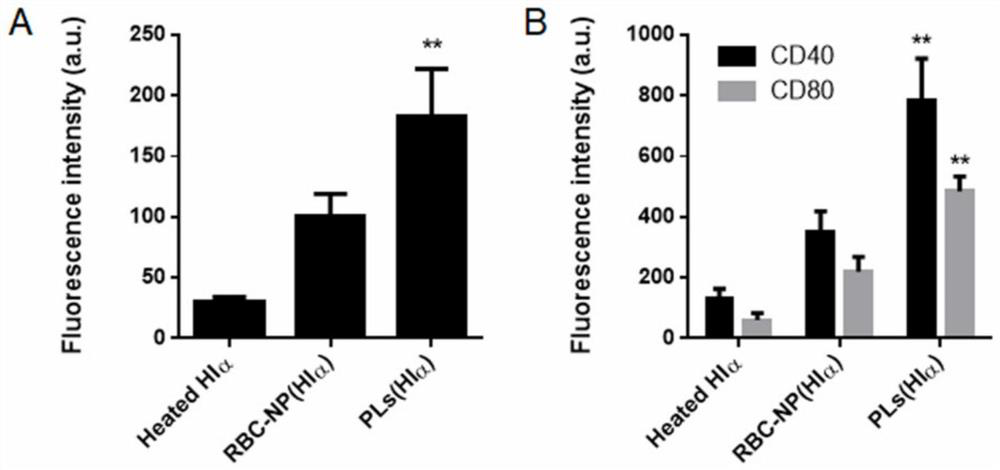
[0045] Next, the in vitro cytotoxicity of PLs (Hlα) was investigated. Specific operation: Extract mouse bone marrow and culture to obtain primary dendritic cells. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com