Rasal2 protein 123-site phosphorylation specific antibody, preparation method and ELISA detection kit
A detection kit and phosphorylation technology, applied in chemical instruments and methods, immunoglobulins, anti-enzyme immunoglobulins, etc., can solve the problems that there is no phosphorylated Rasal2 detection antibody, and achieve inhibition of tumor occurrence and high purity , the effect of promoting drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of Ser123 Phosphorylated Rasal2 Complete Antigen
[0048] ① Design the antigenic peptide phosphorylated at S123 site, such as figure 1 As shown, at the same time, according to the amino acid characteristics of the polypeptide sequence, cysteine (Cys) is added at the front end or the end so that the antigen peptide can be coupled with hemocyanin;
[0049] ② Prepare hemocyanin solution, dissolve hemocyanin into PBS, so that the final concentration is 2mg / ml;
[0050] ③ Mix the hemocyanin solution with the Sulfo-SMCC solution, and incubate at room temperature for 4 hours;
[0051] ④ Dialyze with PBS to remove free SMCC, and calculate the protein concentration by the Broadford method;
[0052] ⑤ Mix the synthesized antigen peptide phosphorylated at S123 with hemocyanin-SMCC solution, and incubate at room temperature for 4 hours to prepare Rasal2 phosphorylated antigen at position 123;
Embodiment 2
[0053] Example 2 Preparation of Rasal2 Phosphorylated Antibody
[0054] ① Phosphorylated complete antigen at the 123rd position of Rasal2 was added with an equal amount of complete Freund's adjuvant and emulsified;
[0055] ② Inject the emulsified antigen into the back of New Zealand rabbits at multiple points intradermally, each dose of 0.5mg / kg per rabbit (basic immunity);
[0056] ③ After 20 days of basic immunization, fully mix and emulsify the above antigen with an equal volume of Freund's incomplete adjuvant;
[0057] ④ Inject the emulsified antigen into the back of New Zealand rabbits at multiple points intradermally for the first booster immunization;
[0058] ⑤ After 20 days, perform the second booster immunization according to the operation steps in ④;
[0059] ⑥ After 14 days of the second booster immunization, the immunized animals were sacrificed and blood was collected. Place the serum in an ice-water bath for 4 hours, centrifuge at 5000 rpm for 10 minutes, an...
Embodiment 3
[0061] Example 3 p-Rasal2 (Ser123) antibody specific identification
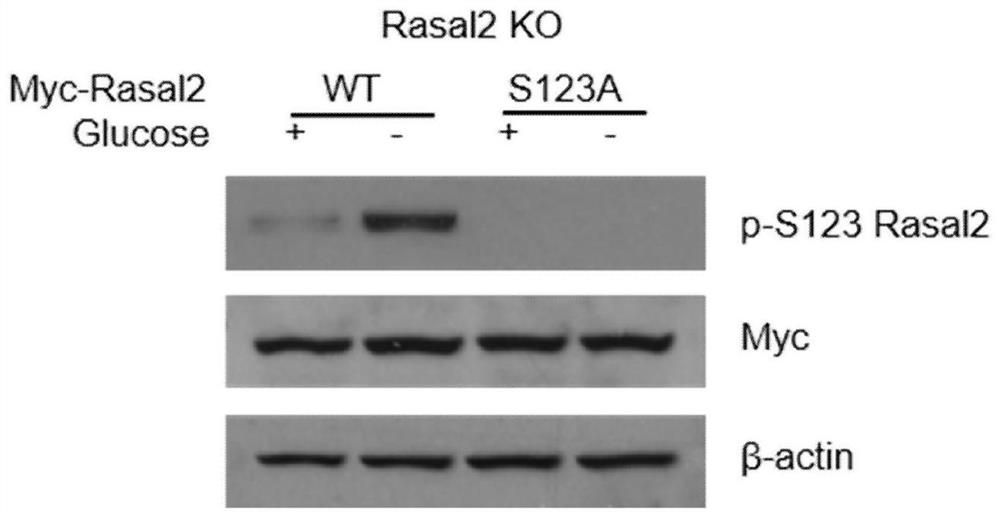
[0062] Construct the Rasal2 S123A mutant, Myc-Rasal2-S123A, and transfer the recombinant vectors Myc-Rasal2-S123A and Myc-Rasal2-WT into 293T cells respectively, then add glucose starvation for 4 hours, and carry out co-immunoprecipitation through M2-Beads, To identify whether the p-Rasal2 antibody can recognize two differently expressed proteins, so as to determine the specificity of the antibody;
[0063] The result is as figure 2 As shown, where WT: 293T cells transfected with Myc-Rasal2-WT expression vector, S123A: 293T cells transformed with point mutation Myc-Rasal2-S123A;
[0064] The experimental results showed that the obtained anti-p-Rasal2 (Ser123) antibody could specifically recognize the phosphorylation at position 123 of Rasal2, and glucose starvation could significantly increase the phosphorylation of Rasal2 (Ser123).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com