Spina date seed capsule and preparation method thereof
A technology of jujube seeds and capsules, which is applied in the directions of capsule delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve the problems of large film-forming water loss, prone to brittle cracking, poor toughness of the capsule shell, etc. The effect of improving performance, dense network structure, and improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
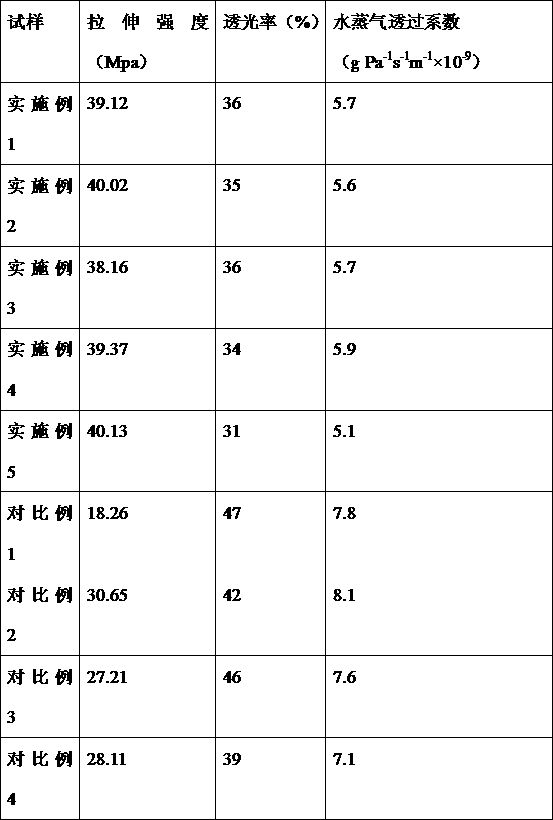
Examples
Embodiment 1
[0023] The raw materials for the core include: Jujube Seed, Angelica, Polygala, Radix Paeoniae Alba, Poria, and Gardenia; and the raw materials for the capsule, which include 28 parts by weight of gelatin, 13 parts by weight of oyster shells, 20 parts by weight of vegetable oil, 0.4 parts of nano grade titanium dioxide and 10 parts by weight of a plasticizer, and the plasticizer is glycerin and triethyl citrate at a mass ratio of 1:1.
[0024] The core medicinal materials are made into decoction pieces (100 parts by weight of Suanzaoren, 90 parts by weight of Angelica sinensis, 90 parts by weight of Polygala, 80 parts by weight of Radix Paeoniae Alba, 70 parts by weight of Poria cocos, 50 parts by weight of Gardenia), add 1.5 times the amount of water, and heat Soak at 40°C for 1 hour, add water to decoct, combine the decoction liquid, filter, concentrate under reduced pressure to a relative density of 1.1 at 80°C, add ethanol to make the alcohol content reach 70%, refrigerate ...
Embodiment 2
[0031] The raw materials for the core include: Jujube Seed, Angelica, Polygala, Radix Paeoniae Alba, Poria, and Gardenia; and the raw materials for the capsule, which include 29 parts by weight of gelatin, 12 parts by weight of oyster shells, 20 parts by weight of vegetable oil, and 0.3 parts of titanium dioxide. and 19 parts by weight of a plasticizer, the plasticizer being glycerin and triethyl citrate at a mass ratio of 1:1.
[0032] The core medicinal materials are made into decoction pieces (100 parts by weight of Suanzaoren, 90 parts by weight of Angelica, 90 parts by weight of Polygala, 80 parts by weight of Radix Paeoniae Alba, 70 parts by weight of Poria cocos, 50 parts by weight of Gardenia), add 12 times the amount of water, and heat Soak at 50°C for 1 hour, add water to decoct, combine the decoction liquid, filter, concentrate under reduced pressure to a relative density of 1.15 at 80°C, add ethanol to make the alcohol content reach 70%, refrigerate and stand still,...
Embodiment 3
[0039] The raw materials for the core include: Jujube Seed, Angelica, Polygala, Radix Paeoniae Alba, Poria, and Gardenia; and the raw materials for the capsule, which include 28 parts by weight of gelatin, 13 parts by weight of oyster shells, 20 parts by weight of vegetable oil, and 0.5 parts of titanium dioxide. And 10 parts by weight of a plasticizer, the plasticizer is glycerin and triethyl citrate at a mass ratio of 1:1.
[0040] The core medicinal materials are made into decoction pieces (100 parts by weight of Suanzaoren, 90 parts by weight of Angelica sinensis, 90 parts by weight of Polygala, 80 parts by weight of Radix Paeoniae Alba, 70 parts by weight of Poria cocos, 50 parts by weight of Gardenia), adding 1.5-2 times the amount of water , heat at 40-50°C, soak for 1 hour, add water to decoct, combine the decoction liquid, filter, concentrate under reduced pressure to a relative density of 1.12 at 80°C, add ethanol to make the alcohol content reach 70%, refrigerate and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com