Folic acid synthesis method
A synthetic method and technology of folic acid, applied in organic chemistry and other fields, can solve problems such as cumbersome post-treatment process, avoid the use of a large amount of hydrochloric acid, have mild reaction temperature, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
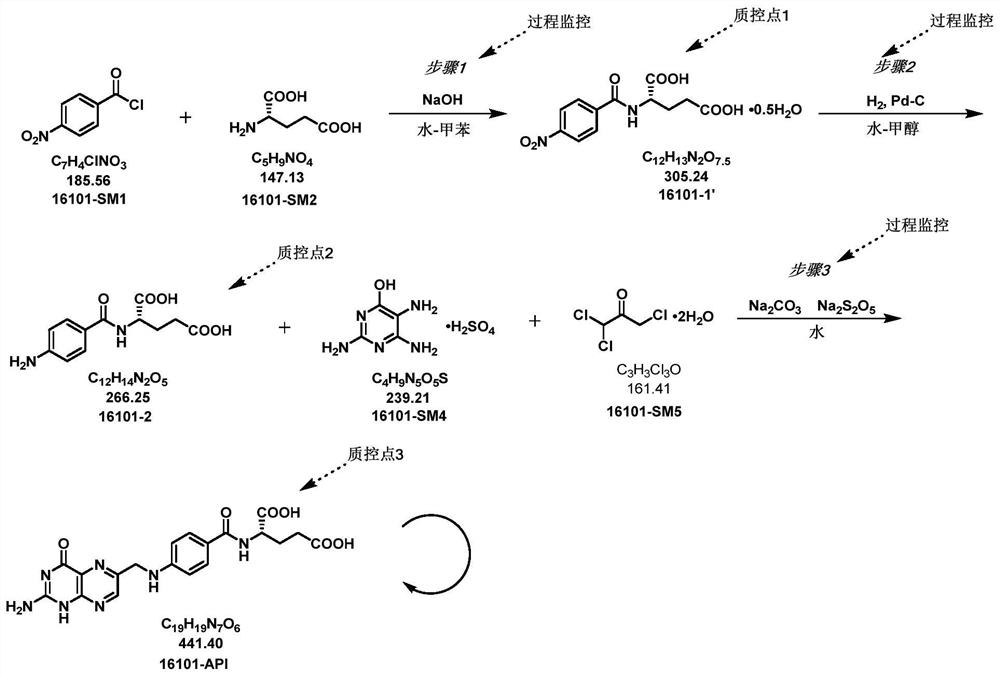
[0041] The embodiment of the present invention provides a folic acid synthesis method, comprising the following steps:
[0042] mixing p-nitrobenzoyl chloride and L-glutamic acid in water, performing a condensation reaction at a pH of 10 to 11, and separating the water phase in the product obtained by the condensation reaction;
[0043] The aqueous phase, ammonium formate and palladium carbon are subjected to a reduction reaction and then the palladium carbon is removed to obtain a reduced product;
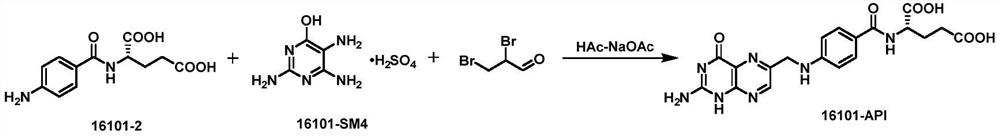
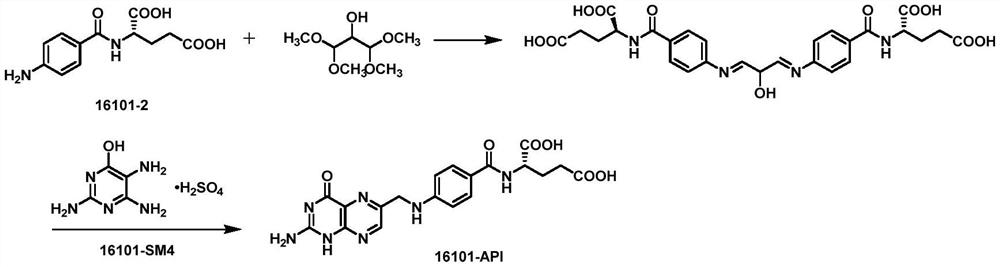
[0044] The reduction product, 2,4,5-triamino-6-hydroxypyrimidine sulfate, 1,1,3-trichloroacetone and pyrosulfous acid are subjected to ring closure reaction under the condition of pH=2-4.
[0045] In some embodiments, in terms of molar ratio, the amount of reaction raw materials added is p-nitrobenzoyl chloride and L-glutamic acid=1: (1-1.1).
[0046] In some embodiments, based on the molar ratio, the addition amount of the reaction raw materials is p-nitrobenzoyl chloride and am...
Embodiment 1
[0067] Put 17.44g (0.12mol) L-glutamic acid and 80mL water into a 500ml reaction bottle, stir and cool down to 5°C, add dropwise a 15% sodium hydroxide solution until the reaction system dissolves, and then simultaneously add p-Nitrate The toluene solution of p-nitrobenzoyl chloride (20.00g (0.11mol) p-nitrobenzoyl chloride is dissolved in 80ml toluene), pH=10 is controlled, and the reaction is dripped for 2h. Phase HPLC intermediate 1 purity 99.46%.
[0068] Put the water phase into a 500ml reaction flask, then add 27.20g (0.43mol) of ammonium formate and 1.28g of palladium carbon, stir and heat up to 50±5°C, react for 3h, filter off palladium carbon, the purity of HPLC intermediate 2 of the filtrate is 99.14 %.
[0069] Put the filtrate into a 1000ml reaction flask, add 600ml of water, under stirring, adjust the pH to 3 ± 0.5 with concentrated hydrochloric acid, then add 30.94g of 2,4,5-triamino-6-hydroxypyrimidine sulfate (0.13mol), 79.53g of 1,1,3-trichloroacetone (0.49m...
Embodiment 2
[0071] Put 15.86g of L-glutamic acid (0.11mol) and 80mL of water into a 500ml reaction bottle, stir and cool down to 10°C, add dropwise a 15% sodium hydroxide solution until the reaction system dissolves, and then add p-Nitrate dropwise at the same time Toluene solution of p-nitrobenzoyl chloride (20.00g p-nitrobenzoyl chloride dissolved in 80ml toluene), control pH = 11, drop the reaction for 2h, leave the reaction solution to separate the liquid, separate the organic phase, the middle of the HPLC of the aqueous phase Body 1 has a purity of 93.87%.
[0072]Put the water phase into a 500ml reaction flask, add 27.20g of ammonium formate and 1.28g of palladium carbon, stir and heat up to 50±5°C, react for 3h, filter off palladium carbon, and the purity of HPLC intermediate 2 of the filtrate is 93.21%.
[0073] Put the filtrate into a 1000ml reaction flask, add 600ml of water, under stirring, adjust the pH to 3 ± 0.5 with concentrated hydrochloric acid, then add 30.94g of 2,4,5-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com