Preparation method of 2-aminomalonamide
A technology of aminomalonamide and diethyl aminomalonate, applied in the field of chemical preparation, can solve the problems of long cycle, low purity and high equipment requirements, and achieve the effects of simple operation, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Pass 24.1g of ammonia gas into 200g of purified water and lower the temperature to 10°C, add dropwise a solution consisting of 100g of diethyl 2-aminomalonate hydrochloride and 100g of purified water, after the addition is complete, at a temperature of 10°C Heat preservation reaction. After the reaction, drop to -8°C to crystallize for 2 hours, filter, and dry at 50°C for 8 hours to obtain 50.89 g of product. During the reaction process, the liquid phase is centrally controlled, and the reaction process is monitored by HPLC method. The monitoring results show that after the dropwise addition, the reaction ends in 6 hours.
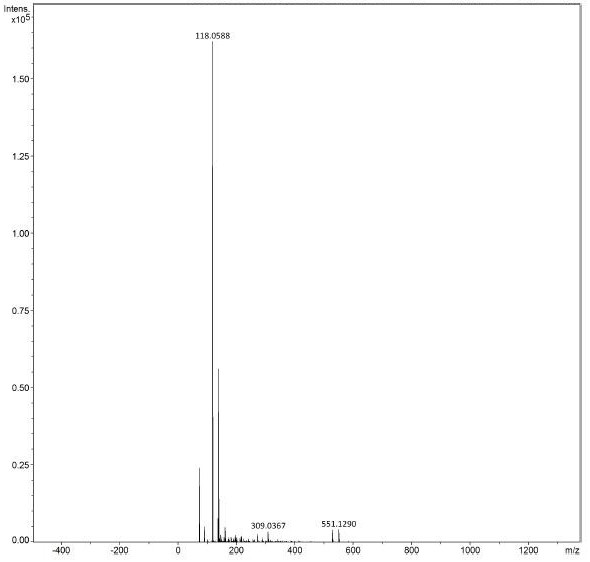
[0047] The obtained product is detected by mass spectrometry, and the detection results are as follows: figure 1 As shown, the molecular weight is 118.0588 (the molecular weight detected by positive ion mass spectrometry is 118.0588, and the actual molecular weight is 117.0588), and the charge-to-mass ratio (m / z) is 117.05. according to figure 1 It...
Embodiment 2
[0051] Pass 150.5g of ammonia gas into 1000g of purified water and cool down to 0°C, add dropwise a solution consisting of 500g of diethyl 2-aminomalonate hydrochloride and 500g of purified water, after the addition is completed, the temperature of 0°C Heat preservation reaction. After the reaction, drop to -5°C to crystallize for 2 hours, filter, and dry at 55°C for 8 hours to obtain 257.25 g of product. During the reaction process, the liquid phase is centrally controlled, and the reaction process is monitored by HPLC. The monitoring results show that after the dropwise addition, the reaction ends in 8 hours.
[0052] The obtained product is detected by mass spectrometry, and it can be confirmed that the obtained product is 2-aminomalonamide according to the detection data.
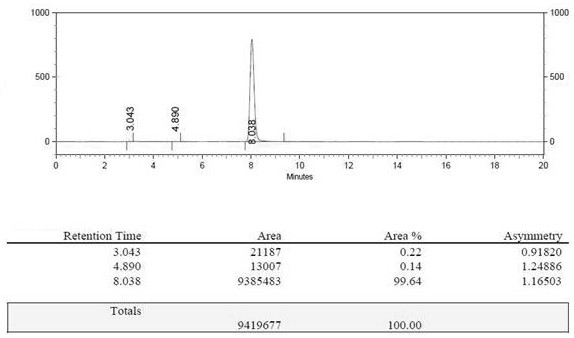
[0053] In terms of 2-aminomalonate diethyl ester hydrochloride, the yield of gained 2-aminomalonamide is 93%, and the prepared 2-aminomalonamide is analyzed by high performance liquid phase, and the detec...
Embodiment 3
[0056] Pass 4.01kg of ammonia gas into 40kg of purified water and lower the temperature to -5°C, add dropwise a solution of 10kg of 2-aminomalonate diethyl hydrochloride and 10kg of purified water, after the dropwise addition, the temperature condition of 5°C The reaction was carried out under heat preservation. After the reaction was completed, the temperature was lowered to -8°C to crystallize for 3 hours, filtered, and dried at 50°C for 8 hours to obtain 5.15 kg of the product. During the reaction process, the liquid phase is centrally controlled, and the reaction process is monitored by HPLC method. The monitoring results show that after the dropwise addition, the reaction ends in 7 hours.
[0057] The obtained product is detected by mass spectrometry, and it can be confirmed that the obtained product is 2-aminomalonamide according to the detection data.
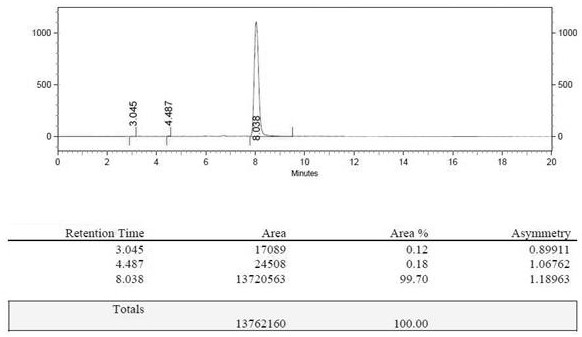
[0058] In terms of 2-aminomalonate diethyl ester hydrochloride, the yield of gained 2-aminomalonamide is 93.1%, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com