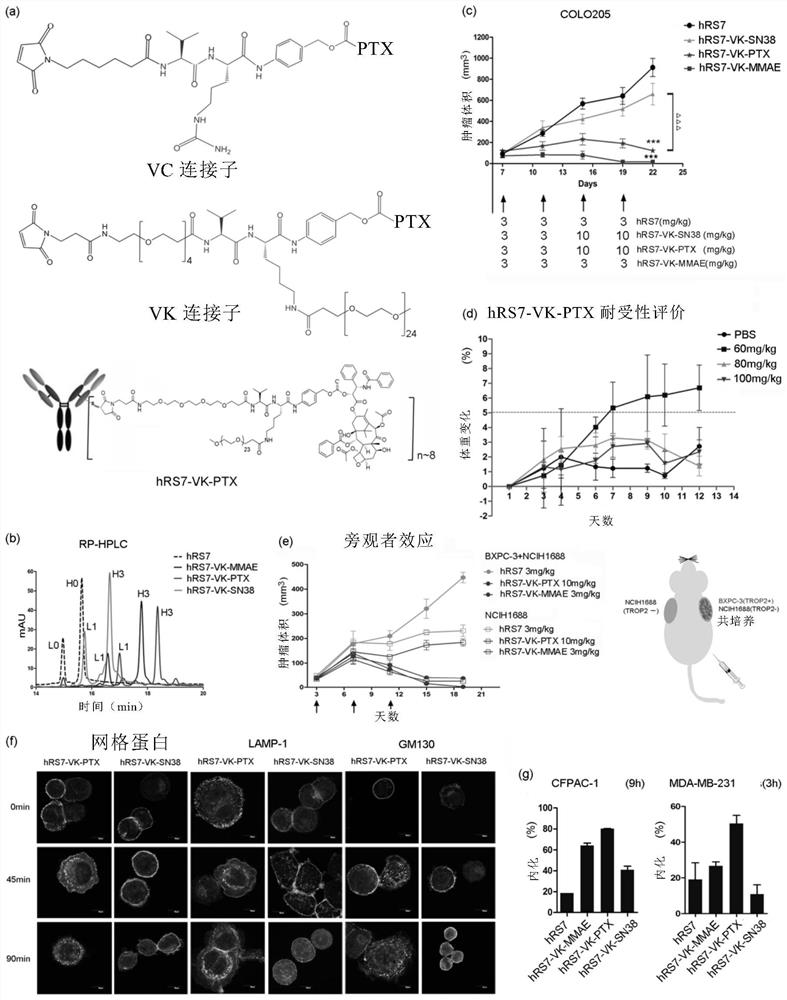
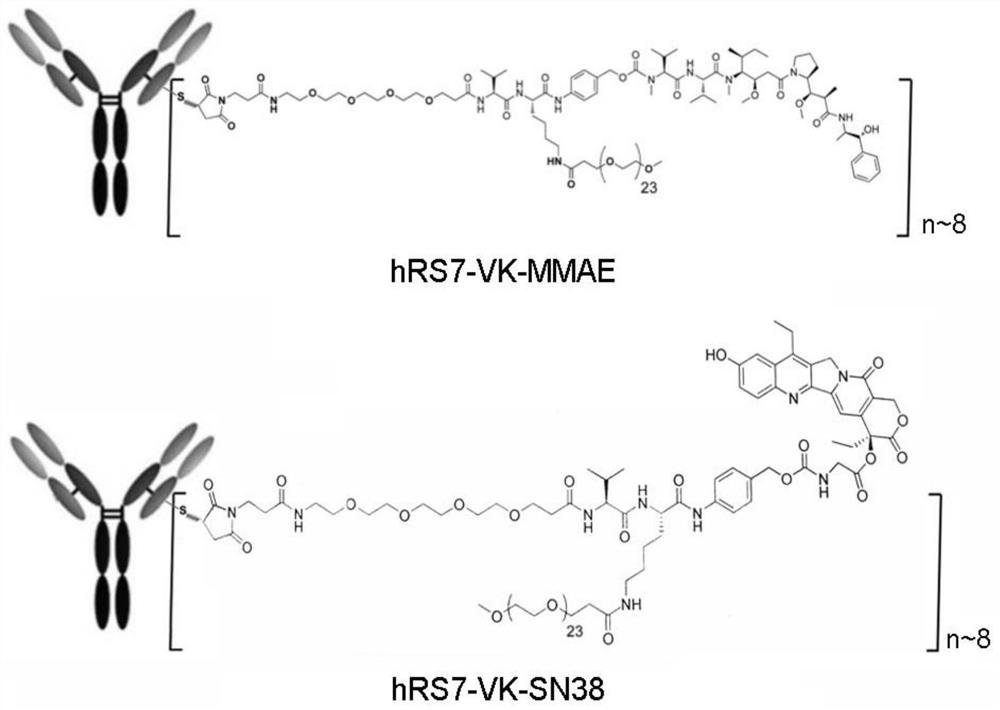
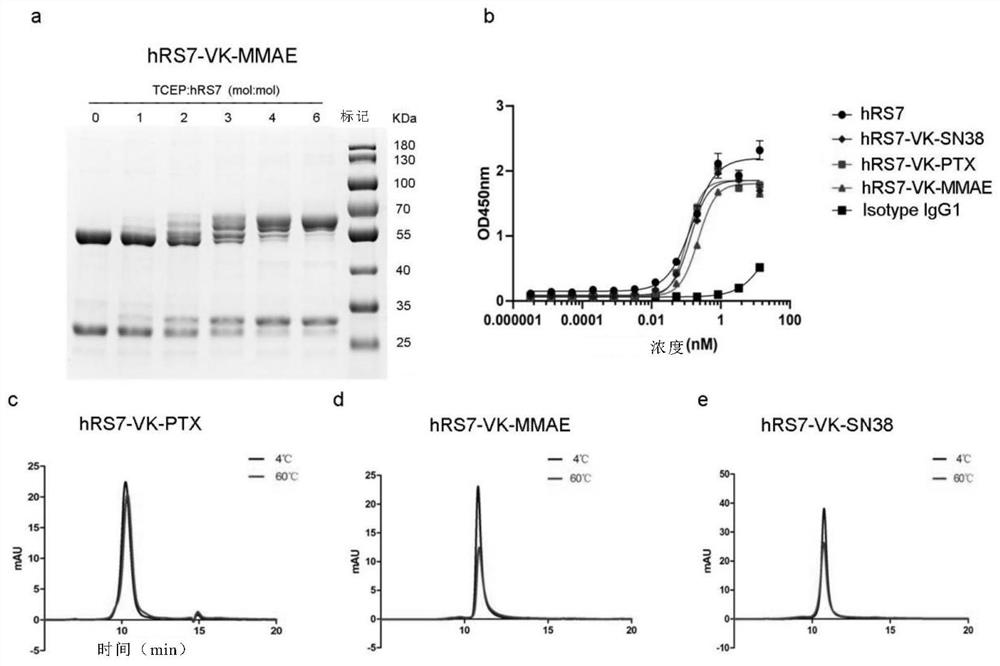
Intermediate for preparing antibody and drug conjugate (ADC) and preparation method and application thereof
A technology of conjugated body and antibody, applied in the field of medicine and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0208] (b) Preparation of ADC and detection of DAR value
[0209] The reduction reaction was carried out by adding 6 molar equivalents of TCEP (1 mg / mL) to 8 mg / mL antibody solution containing 9% sucrose, 10 mM acetate, 0.01% Tween-20, pH 5.0 at 37°C for 2 hours. After reduction, 20 molar equivalents of the linker payload were added per antibody sulfhydryl group and incubated at 4°C for more than 16 hours. The solution mixture was concentrated by centrifugation (4000 rpm, 15 minutes, Thermo Fisher ST40R TX-1 ) with an Amicon Ultracontainer (50,000 MWCO, Millipore Corporation). The concentration of ADC was measured by UV detector (Nanodrop 100, Thermo Fisher Scientific Inc.) under absorption at 280 nm. The DAR value of the final product was confirmed by reverse phase (RP)-HPLC and QTOF mass spectrometer.
[0210] (c) RP-HPLC analysis of DAR value of ADC
[0211] RP-HPLC analysis is carried out under the following conditions on 1260Infinity UHPLC (Agilent Technologies): 1) Ag...
Embodiment 1
[0234] Antibody preparation
[0235] In this example, hRS7 antibody was expressed and purified to obtain hRS7 antibody. Methods as below:
[0236] The amino acid sequences of hRS7 light and heavy chains were obtained from US Patent No. 9931417B2, and then reverse-transcribed into cDNA sequences by Vector NTI software. The DNA sequence was synthesized and subcloned into the pcDNA3.1 vector and amplified in E. coli. The purified plasmid was transfected into HEK293 cells by PEI. Then suspend the cells in FreeStyle TM293 expression medium. After 5 days of culture, cell culture supernatants were harvested and antibodies were affinity purified by protein A.
Embodiment 2
[0238] Preparation of Compound VK-PTX
[0239] The structure of compound VK-PTX is shown below:
[0240]
[0241] Paclitaxel was reacted with the VK linker to form the compound VK-PTX, namely
[0242] Mal-peg4-Val-Lys(peg24)-PAB-paclitaxel (VK-PTX), the method is as follows:
[0243]
[0244] Step 1: Synthesis of Compound 3 (Fmoc-Val-Lys(Trt)-PAB-PTX)
[0245] Fmoc-Val-Lys(Trt)-PAB-PNP (purchased from Levena Biopharma, USA) and PTX (purchased from Levena Biopharma, USA) were dissolved in anhydrous DMF, and DIEA was added to the solution, and the mixture was heated at room temperature at 22°C The reaction was stirred for 2 hours, and the target product Fmoc-Val-Lys(Trt)-PAB-PTX was directly purified by reverse HPLC, and compound 3 was formed as a white powder after lyophilization.
[0246] Step 2: Synthesis of compound 4 (Fmoc-Val-Lys-PAB-PTX TFA salt)
[0247] The compound 3 obtained in step 1 was dissolved in 10% TFA / DCM solution and stirred at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com