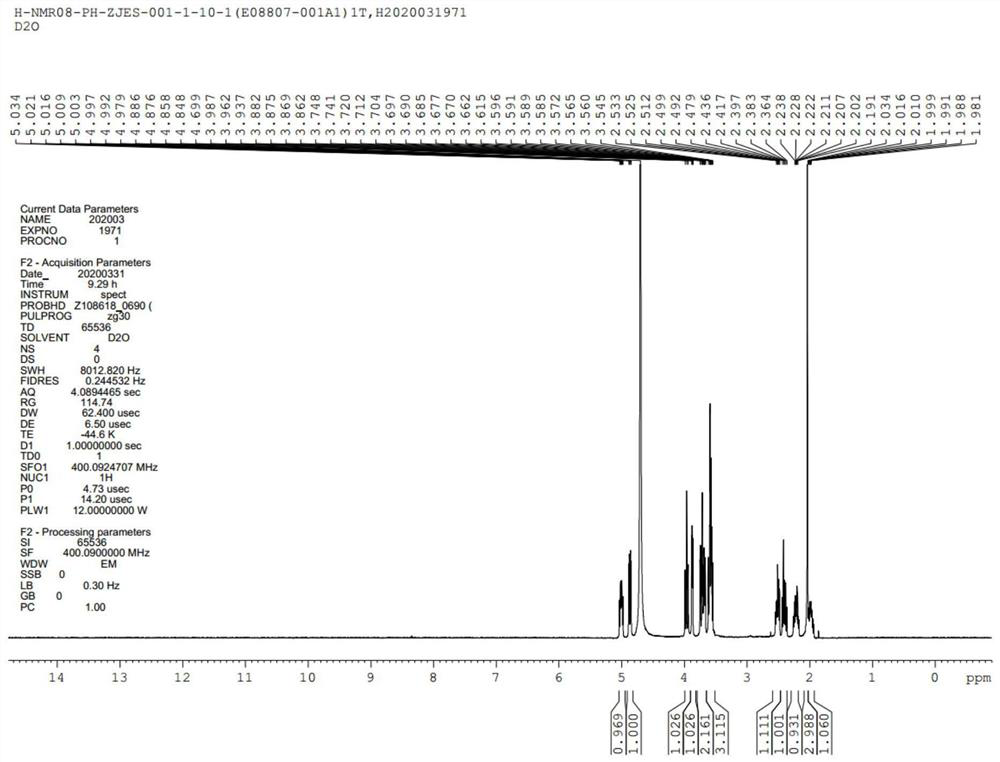
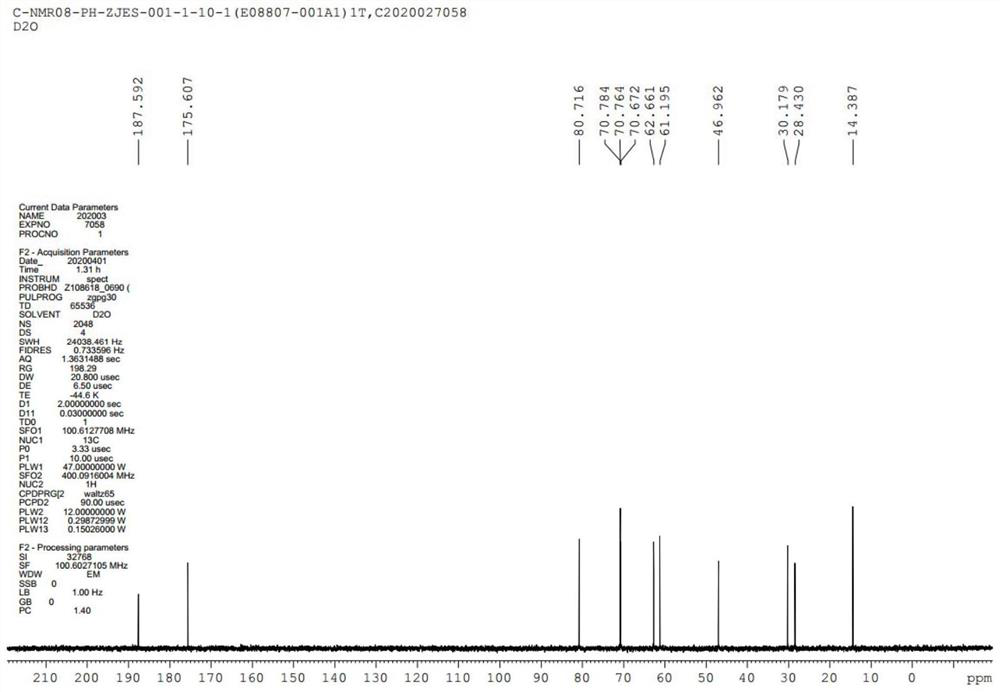
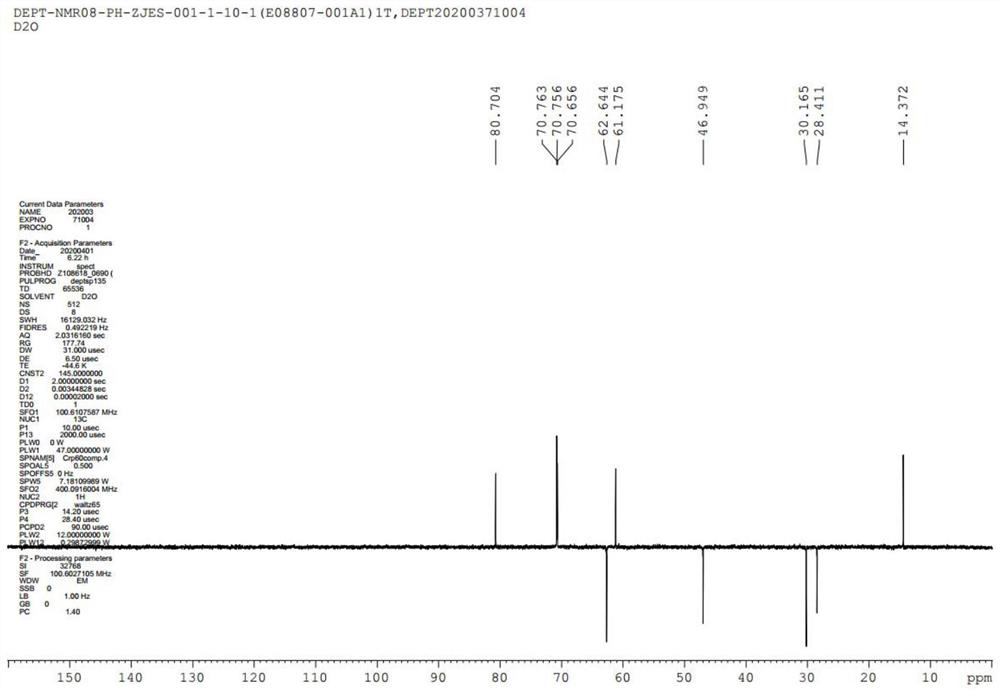
GMDTC drug impurity and preparation method thereof
An impurity and drug technology, applied in the field of GMDTC drug impurities and its preparation, can solve the problems of toxic and side effects, affecting drug efficacy, affecting the purity of GMDTC drugs, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation method of the GMDTC drug impurity of the present embodiment, comprises the following steps:
[0063] (1) Add 200mL of methanol into a 500mL three-necked flask, and add 20g of sodium hydroxide with stirring, and stir until the solid is basically dissolved completely;
[0064] (2) Add 74.5g of L-methionine, stir until the solid is basically dissolved completely; add 90g of D-(+)-glucose at one time, and react at 25°C for 6h;
[0065] (3) Cool down to 0°C, add 100mL of water dropwise; add 37g of sodium borohydride in batches, control the temperature at 30°C for 10-20h;
[0066] (4) Cool down to 0°C, add hydrochloric acid to adjust the pH of the system to 1; crystallize at room temperature, filter, and wash the filter cake; dry, recrystallize, dry, and collect 71.04 g of white solid;
[0067] (5) Add 300 mL of purified water and 31.3 g of the white solid obtained in step (4), stir, add 62.6 g of sodium carbonate at room temperature, and stir until the solid...
Embodiment 2
[0071] The preparation method of the GMDTC drug impurity of the present embodiment, comprises the following steps:
[0072] (1) Add 250mL of methanol into a 500mL three-neck flask, and add 20g of sodium hydroxide with stirring, and stir until the solid is basically dissolved completely;
[0073] (2) Add 74.5g of L-methionine, stir until the solid is basically dissolved completely; add 100g of D-(+)-glucose at one time, and react at 30°C for 15h;
[0074] (3) Cool down to 5°C, add 100mL of water dropwise; add 50g of sodium borohydride in batches, and react at 35°C for 15h;
[0075] (4) Cool down to 2°C, add hydrochloric acid to adjust the pH of the system to 2; crystallize at room temperature, filter, and wash the filter cake; dry, recrystallize, dry, and collect 82.15 g of white solid;
[0076] (5) Add 300 mL of purified water and 31.3 g of the white solid obtained in step (4), stir, add 70 g of sodium carbonate at room temperature, and stir until the solid dissolves complete...
Embodiment 3
[0080] The preparation method of the GMDTC drug impurity of the present embodiment, comprises the following steps:
[0081] (1) Add 300mL of methanol into a 500mL three-neck flask, and add 20g of sodium hydroxide with stirring, and stir until the solid is basically dissolved completely;
[0082] (2) Add 74.5g of L-methionine, stir until the solid is basically dissolved completely; add 120g of D-(+)-glucose at one time, and react at 35°C for 24h;
[0083] (3) Cool down to 10°C, add 100mL of water dropwise; add 74g of sodium borohydride in batches, and react at 40°C for 20h;
[0084] (4) Cool down to 5° C., add hydrochloric acid to adjust the pH of the system to 3; crystallize at room temperature, filter, and wash the filter cake; dry, recrystallize, and dry to obtain 80.19 g of white solid;
[0085] (5) Add 300 mL of purified water and 31.3 g of the white solid obtained in step (4), stir, add 75 g of sodium carbonate at room temperature, and stir until the solid dissolves comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com