Method for separating and detecting 1, 2-propylene glycol enantiomer by gas chromatography
A technology of enantiomers and propylene glycol, applied in the field of analysis and detection, can solve the problems of inability to accurately quantify trace impurities, insufficient separation ability, poor chromatographic peak shape, etc., to solve the problem of drug quality control, ensure effectiveness and safety , the effect of less interference factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 detection method effectiveness verification
[0052] Sample preparation:
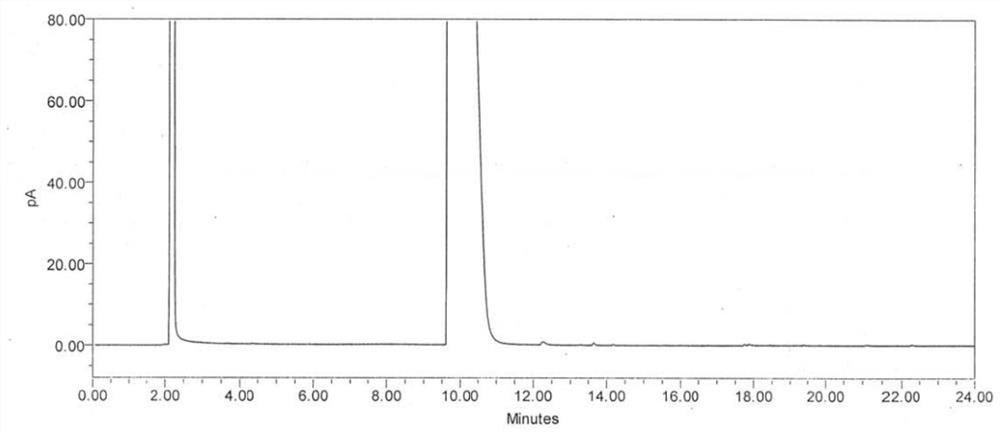
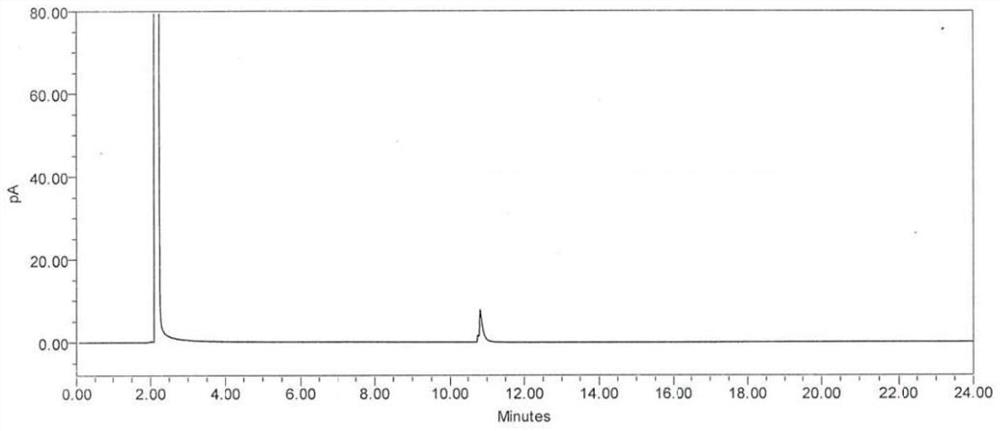
[0053] Weigh 200mg of (S)-1,2-propanediol, place it in a 20ml measuring bottle, dilute to volume with absolute ethanol, shake well, and use it as the test solution 1.
[0054] Weigh 50mg of (R)-1,2-propanediol, place it in a 10ml measuring bottle, dilute to the mark with absolute ethanol, shake well, measure 1ml and place it in a 10ml measuring bottle, use Dilute to volume with water and ethanol, shake well, and use as test solution 2.
[0055] Weigh 40 mg of (S)-1,2-propanediol, weigh it accurately, place it in a 10ml Nessler colorimetric tube or a 10ml centrifuge tube, add 200 μl of acetic anhydride and 10 μl of methanesulfonic acid, vortex and mix for 10 minutes, add Saturated sodium carbonate solution 3ml, after vortex mixing for 30 seconds, add 5ml of n-heptane for extraction, shake vigorously to make full contact, static layering, take the supernatant as the test solution 3. ...
Embodiment 2-5
[0064] Example 2-5 Derivatization reaction condition optimization
[0065] Take 40mg of (S)-1,2-propanediol, put it in a 10ml Nessler colorimetric tube or a 10ml centrifuge tube, add the derivatizing agent acetic anhydride and catalyst methanesulfonic acid respectively according to Table 1, vortex and mix for a certain period of time, Add 3ml of quenching agent (water, saturated sodium chloride solution or saturated sodium carbonate solution), vortex and mix for 30 seconds, add 5ml of n-heptane for extraction, shake vigorously to make full contact, static layering, take the supernatant as The test solution.
[0066] Get above-mentioned need testing solution, carry out gas chromatograph analysis according to the chromatographic condition of need testing solution 3 or 4 of embodiment 1. The results are shown in Table 1.
[0067] Table 1
[0068]
[0069]
Embodiment 6
[0070] Embodiment 6 detection method specific verification
[0071] Sample preparation:
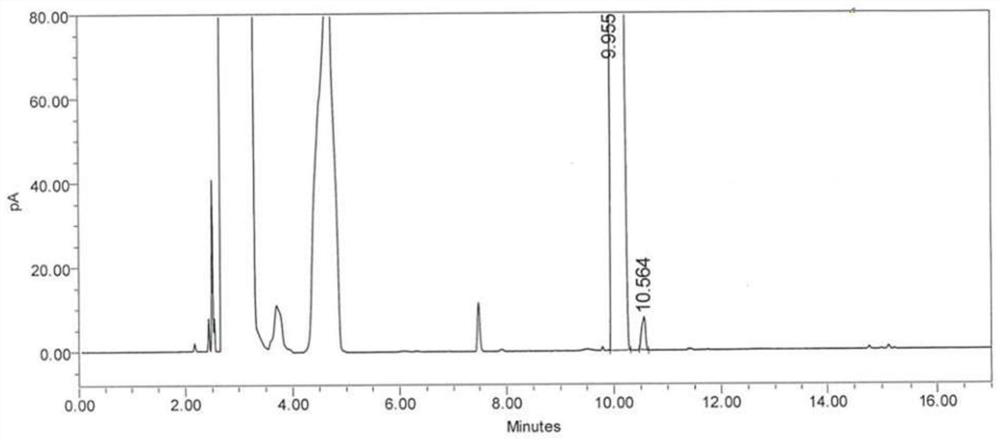
[0072] 1. Take about 40mg each of (S)-1,2-propanediol and (R)-1,2-propanediol, put them in a 10ml Nessler colorimetric tube or a 10ml centrifuge tube, add 200μl of acetic anhydride and 10μl of methanesulfonic acid respectively , vortex and mix for 10 minutes, add 3ml of saturated sodium carbonate solution, vortex and mix for 30 seconds, add 5ml of n-heptane for extraction, static layering, take the supernatant, respectively as solution (1) and solution (2), according to The volume ratio is about 100:1. Measure the solution (1): solution (2) and mix them evenly. The resulting mixed solution is used as the resolution solution.
[0073] 2. Take solution (1) as (S)-1,2-propanediol derivative solution positioning solution; take solution (2) and dilute 100 times as (R)-1,2-propanediol derivative solution positioning solution.
[0074] 3. Take 10ml blank Nessler colorimetric tube or 10ml blank...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com