Avanafil impurity D as well as synthesis method and application thereof
A technology of avanafil and synthesis method, which is applied in the field of organic synthesis and can solve the problems of efficacy and safety impact of the final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
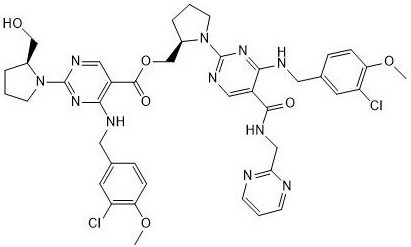
[0020] Example 1: Avanafil is synthesized by dehydration esterification reaction with carboxylic acid Avanafil impurity D
[0021] synthetic route:
[0022] .
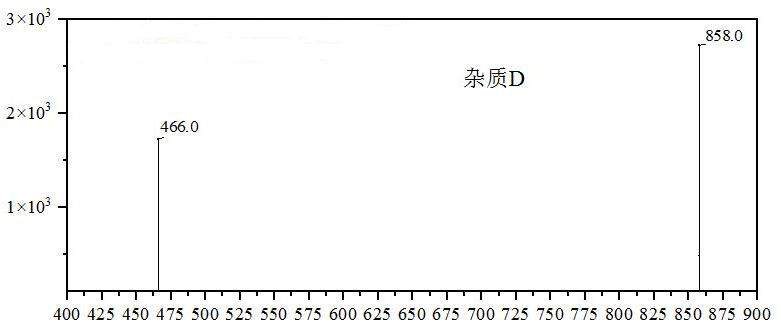
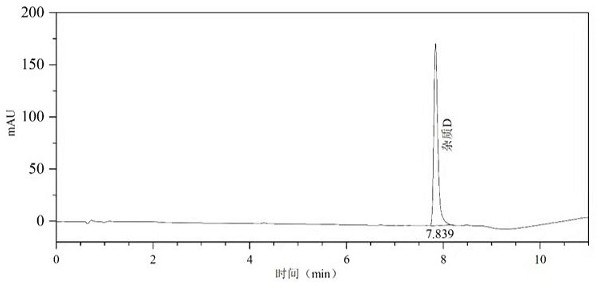
[0023] Synthesis process: Add dichloromethane, avanafil, EDCI and 4-{[(3-chloro-4-methoxyphenyl)methyl]amino}-2-[(2S)-( Hydroxymethyl) pyrrolidin-1-yl] pyrimidine-5-carboxylic acid, after reacting for 3-7h, the reaction solution was quenched, and the oil was obtained after post-treatment, which contained about 20% avanafil by liquid phase detection Impurities D. The oil was purified on the preparative apparatus and evaporated to dryness to obtain avanafil impurity D as a pale yellow solid.
Embodiment 2
[0024] Embodiment 2: Avanafil impurity D and synthetic route are as follows:
[0025] .
[0026] Synthesis of M1: SM2, appropriate amount of H 2 O was added to the three-necked flask, stirred to dissolve and then added excess Na 2 CO 3 , heated in a water bath to 20-30°C, after dissolving, add 2 equivalents of SM1 dropwise, the system is light yellow-green, a large amount of solids precipitate out, stir overnight. The pH was adjusted with concentrated hydrochloric acid to generate a large amount of gas, filtered and washed, and the filter cake was air-dried to obtain a white solid with a yield of 84%.
[0027] Synthesis of M2: Add M1 and toluene into a three-necked flask, raise the temperature to 90-100°C, and add excess POCl dropwise 3 , After heating up to 90°C, a yellow turbid solution was obtained, reacted for 2-3h, and gradually cooled down to room temperature. Drop water into the reaction solution, speed up the stirring, stand to separate the layers, separate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com