Method for removing cadmium in wastewater and recovering calcium and cadmium
A recovery method and waste water technology, applied in chemical instruments and methods, water pollutants, multi-stage water treatment, etc., can solve problems such as chronic poisoning of organisms, high toxicity of cadmium compounds, etc., and achieve separation recovery and resource reuse , Calcium and cadmium element recovery is convenient, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare 2mg / L of Cd 2+ Solution, take 500mL of the solution, add 2.22g of calcium chloride and 2.3g of sodium carbonate, react at room temperature for 2h, separate the solid and liquid of the reaction product, and obtain a solid product and the remaining cadmium-containing solution.
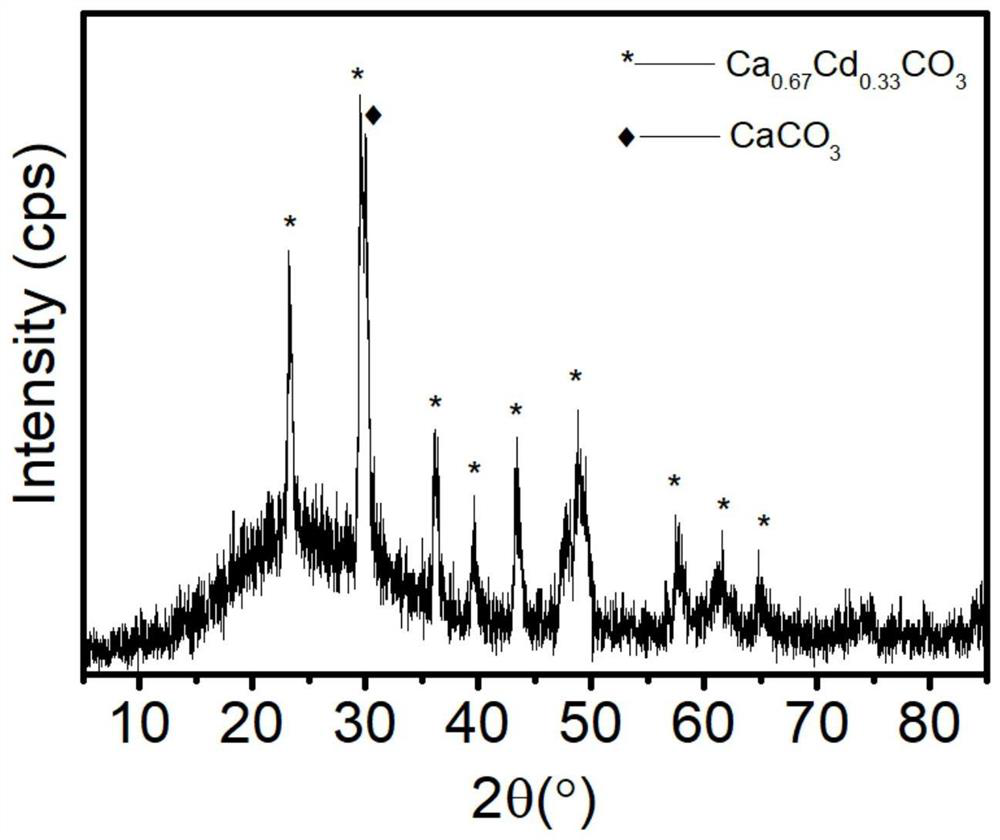
[0046]Take 5 mL of the remaining cadmium-containing solution, filter it through a 0.22 μm syringe filter, and send the clarified solution to an inductively coupled plasma optical emission spectrometer (ICP-OES) for detection. The concentration of cadmium ions in the remaining cadmium-containing solution is 0.0014 mg / L, and Cd 2+ The removal efficiency is 99.93%. After the solid product is dried, X-ray powder diffraction (XRD) is carried out for phase detection, and the results are as follows: figure 1 As shown, the phase is consistent with the standard card 01-072-1938 and 01-085-1108, and the solid powder is Ca 0.67 CD 0.33 CO 3 and CaCO 3 mixture.
Embodiment 2
[0048] Prepare 10mg / L of Cd 2+ Solution, take 50mL of the solution, add 0.222g of anhydrous calcium chloride and 0.320g of sodium hydroxide, react at room temperature for 2 hours, separate the solid and liquid of the reaction product, and obtain a solid product and the remaining cadmium-containing solution.
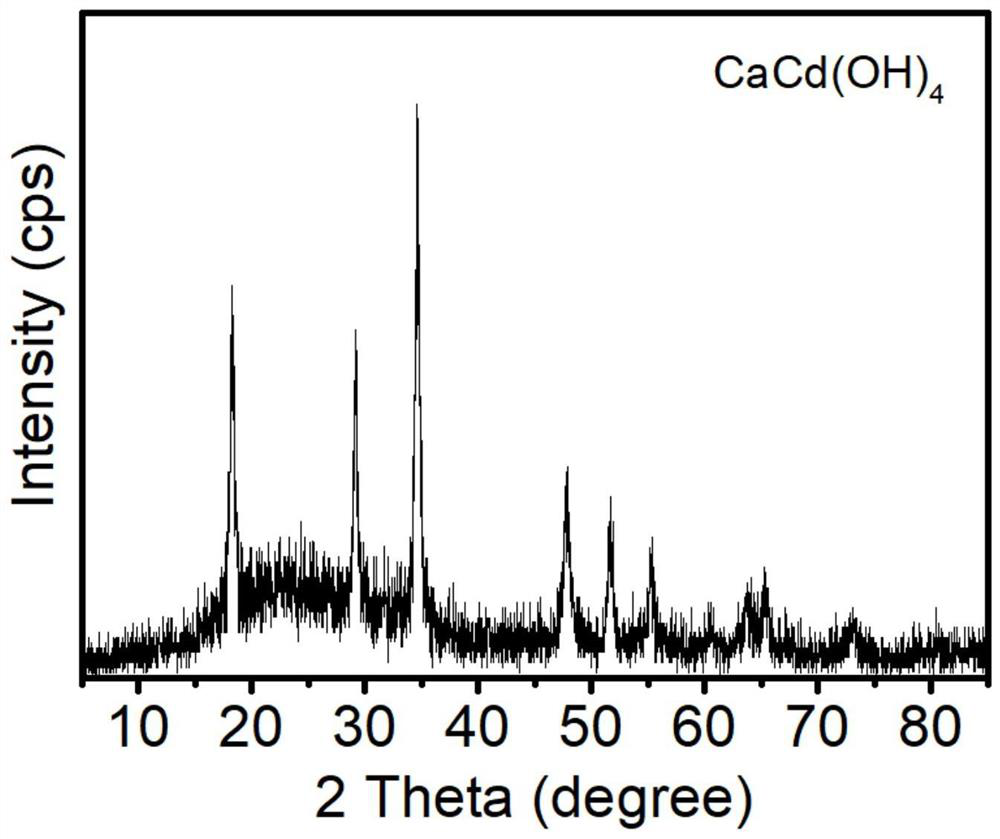
[0049] Take 5mL of the remaining cadmium-containing solution, filter it through a 0.22μm syringe filter, and send the clarified solution to an inductively coupled plasma optical emission spectrometer (ICP-OES) for detection. The concentration of cadmium ions in the remaining cadmium-containing solution is 0.038mg / L, Cd 2+ The removal efficiency is 99.62%. After the solid product is dried, X-ray powder diffraction (XRD) is carried out for phase detection, and the results are as follows: figure 2 As shown, the phase is consistent with the standard card 00-050-02468, and the solid powder is CaCd(OH) 4 .
Embodiment 3
[0051] Prepare 1000mg / L of Cd 2+ Solution, take 100mL of the solution, add 2.22g of anhydrous calcium chloride and 4.48g of potassium hydroxide, stir and react for 1d, and separate the solid and liquid of the reaction product to obtain a solid product and the remaining cadmium-containing solution.
[0052] Take 5 mL of the remaining cadmium-containing solution, filter it through a 0.22 μm syringe filter, and send the clarified solution to an inductively coupled plasma optical emission spectrometer (ICP-OES) for detection. The concentration of cadmium ions in the remaining cadmium-containing solution is 0.74 mg / L, and Cd 2+ The removal efficiency can reach 99.93%. Add anhydrous calcium chloride and potassium hydroxide to the remaining solution until Cd 2+ The detected concentration is lower than 0.05mg / L.
[0053] Add hydrochloric acid to the obtained solid precipitate to completely dissolve the precipitate at room temperature to obtain a clear solution with high concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com