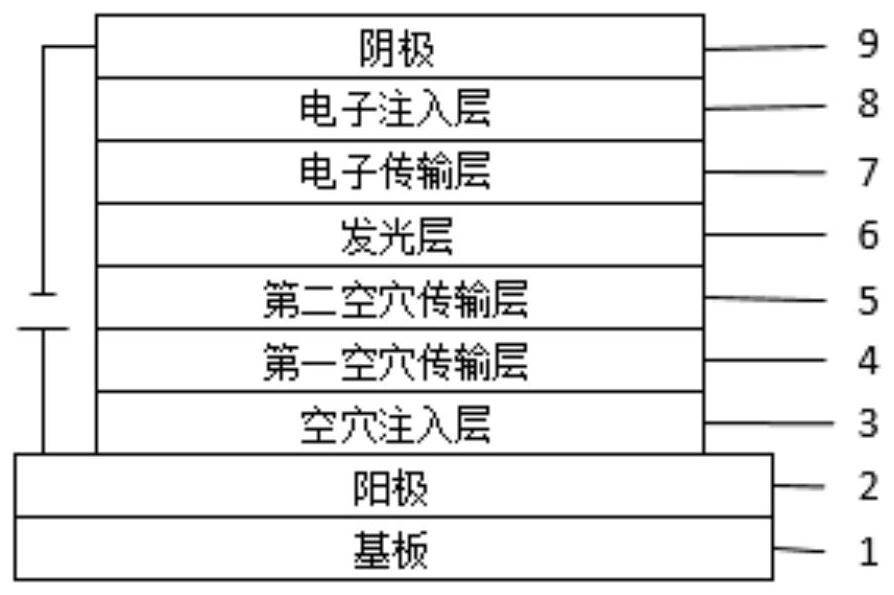
Aromatic amine compound, mixture, composition and organic electronic device
A compound and aromatic amine technology, which is applied in the field of aromatic amine compounds, compositions, organic electronic devices and mixtures, can solve problems such as hole and electron transport imbalance, short device life, etc., to reduce the roll-off effect and drive voltage , The effect of reducing manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Embodiment 1 synthetic compound 1
[0186]
[0187] Synthesis of Intermediate 1-2: Intermediate 1-1 (10.0 g) was dissolved in dichloromethane (100 ml). Triethylamine (8.1 g) was slowly added dropwise in a nitrogen atmosphere at 0°C and stirred for 30 min; then trifluoromethanesulfonic anhydride (22.5 g) was added dropwise and kept stirring at 0°C for 5 h. Then wash with saturated sodium carbonate solution, separate the layers, and collect the organic phase. The solvent was removed by rotary evaporation of the organic phase, followed by column chromatography to obtain intermediate 1-2. MS (ASAP): 321.
[0188] Synthesis of Intermediate 1-4: Dissolve Intermediate 1-2 (14.8g) and Intermediate 1-3 (10.2g) in a mixed solvent of 1,4-dioxane and water (210 / 20ml), And add Pd(PPh 3 ) 4 (0.5g) and potassium carbonate (19.1g). Under nitrogen atmosphere, stir at 100°C for 6h. After cooling, most of the solvent was removed by rotary evaporation, and then extracted with dic...
Embodiment 2
[0192] Embodiment 2 synthetic compound 2
[0193]
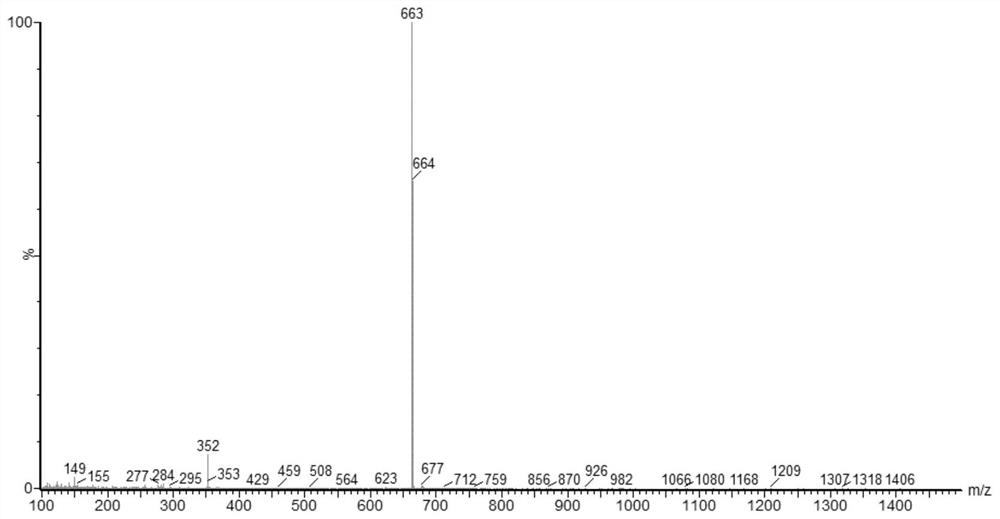
[0194] Synthesis of Compound 2: Intermediate 1-7 (4.70g), Compound 2-1 (2.34g), Pd(dba) 2 (0.10g), TTBP (0.18g) and sodium tert-butoxide (1.50g) were dissolved in toluene and stirred at 75°C for 4h under a nitrogen atmosphere. After cooling, it was washed with water and separated, the organic phase was collected, and the organic phase was rotary evaporated to remove the solvent. The obtained crude product was subjected to column chromatography and recrystallization to obtain compound 2. MS (ASAP): 670.
Embodiment 3
[0195] Embodiment 3 synthetic compound 3
[0196]
[0197] Synthesis of intermediate 3-2: Dissolve compound 3-1 (6.0g) and o-bromoiodobenzene (8.0g) in a mixed solvent of 1,4-dioxane and water (150 / 30ml), and add Pd(PPh 3 ) 4 (0.20g) and potassium carbonate (7.8g). Under nitrogen atmosphere, stir at 100°C for 6h. After cooling, remove most of the solvent by rotary evaporation, then extract the product with dichloromethane and wash it three times with water, separate the liquids, collect the organic phase, remove the solvent by rotary evaporation of the organic phase, and then undergo column chromatography and recrystallization to obtain the intermediate 3-2. MS (ASAP): 323.
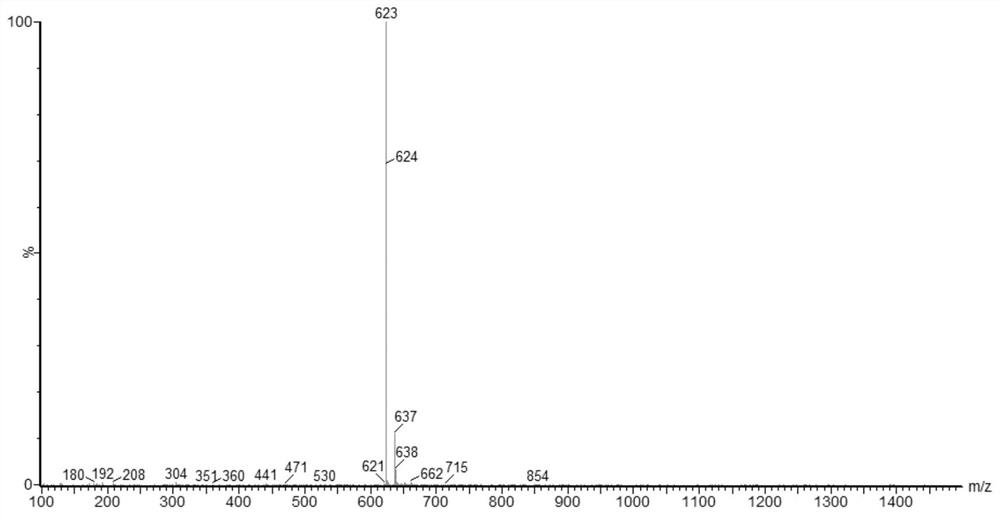
[0198] The synthesis of intermediate 3-3 refers to the synthesis of compound 2 in Example 2, except that intermediate 1-7 is replaced by intermediate 1-5. MS (ASAP):478.
[0199] The synthesis of compound 3 refers to the synthesis of compound 2, except that 1-7 is replaced by 3-3, and 2-1 is rep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com