Primer pair, amplification reagent, kit and detection method for detecting infectious hypodermal and hematopoietic necrosis virus (IHHNV) of prawns and application
A technique for hematopoietic tissue and necrosis virus, which is applied in the field of primer pairs for detecting infectious subcutaneous and hematopoietic tissue necrosis virus of prawns, can solve the problems of unfavorable rapid detection, large instrument dependence, long time consumption, etc., and achieves short detection time and low cost. , The effect of the simple structure of the device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Raw material source:
[0049] Primers and probes were designed and synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.;
[0050] RPA reaction solution and RPA lyophilized powder were purchased from TwistDx Inc, UK.
[0051] The RPA freeze-dried powder includes three enzymes: recombinase proteins uvs X and uvs Y, single-chain binding protein gp32 and Bsu DNA polymerase.
[0052] Establishment of fluorescent RPA detection kit and method for shrimp infectious subcutaneous and hematopoietic necrosis virus:
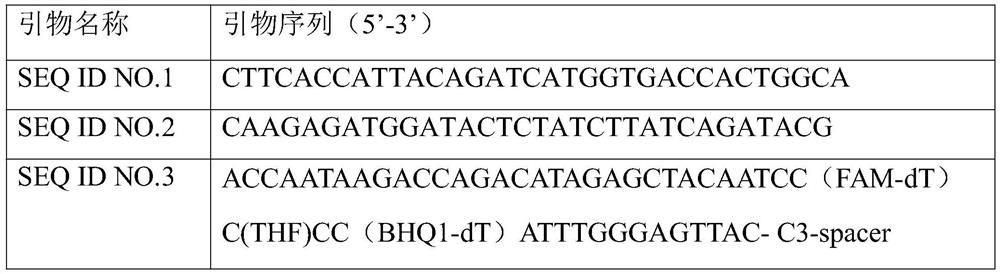
[0053] 1. Design and preparation of primers and probes
[0054] According to the comparison and analysis of the nonstructural protein 1 and nonstructural protein 2 region gene sequences of AF218266.2, EF633688.1, and AY355307.1, the conserved extension region sequence was determined, and the primer probe was designed according to the RPA primer probe design principle. Primer probes were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. Design primer pa...
Embodiment 2
[0068] Sensitivity evaluation of IHHNV fluorescent RPA detection
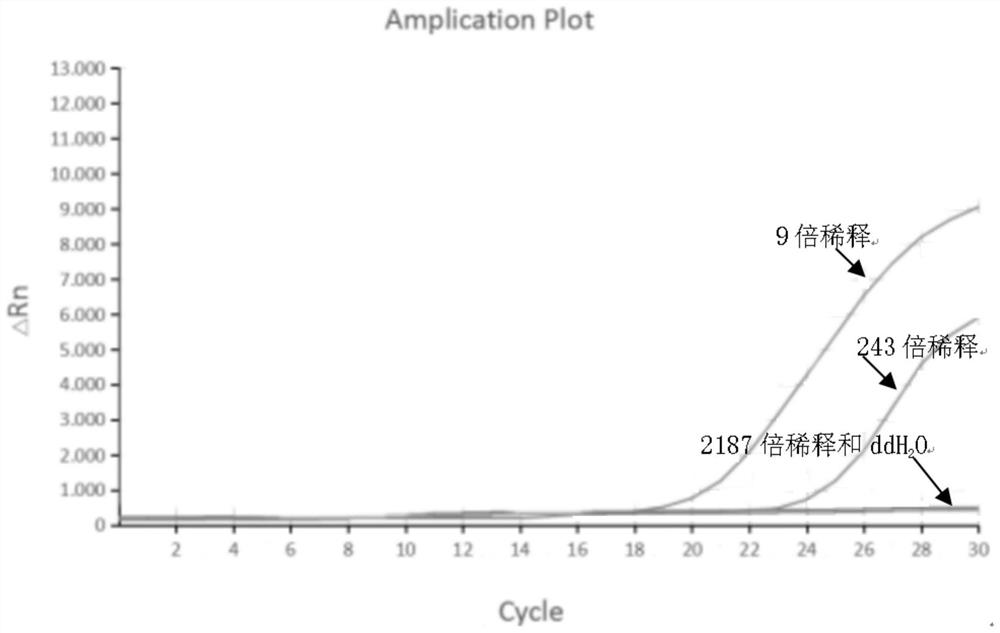
[0069] Use IHHNV standard substance (initial concentration of 12.5ng / ul) to perform 3-fold dilution, select samples with 9-fold, 243-fold, and 2187-fold concentration for detection, and use ddH at the same time 2 O served as a negative control. The result is as figure 1 As shown, 9 times, 243 times the concentration of the sample detection results are positive, 2187 times the concentration of the sample and ddH 2 O tested negative.
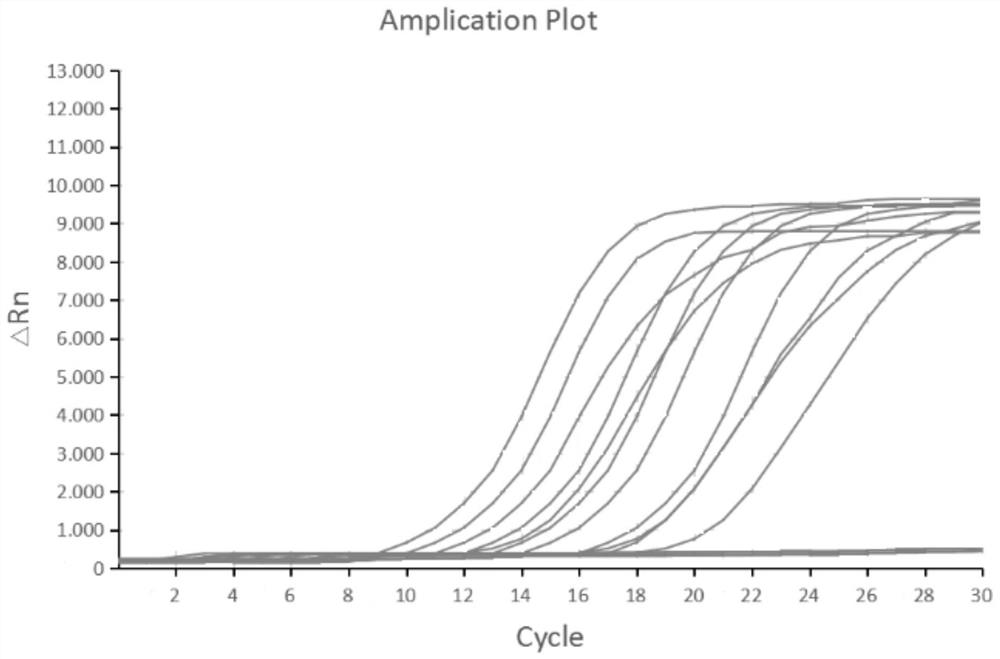
[0070] In order to test the accuracy of the detection method and the effectiveness of clinical application, a batch of clinical samples were detected by this method and the fluorescent PCR method. For the amplification results of the IHHNV fluorescent RPA detection system, see figure 2 , and the comparison chart between the detection results of clinical samples and fluorescent PCR is shown in Table 2 below.
[0071] Table 2 Comparison of IHHNV fluorescent RPA clinical sample ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com